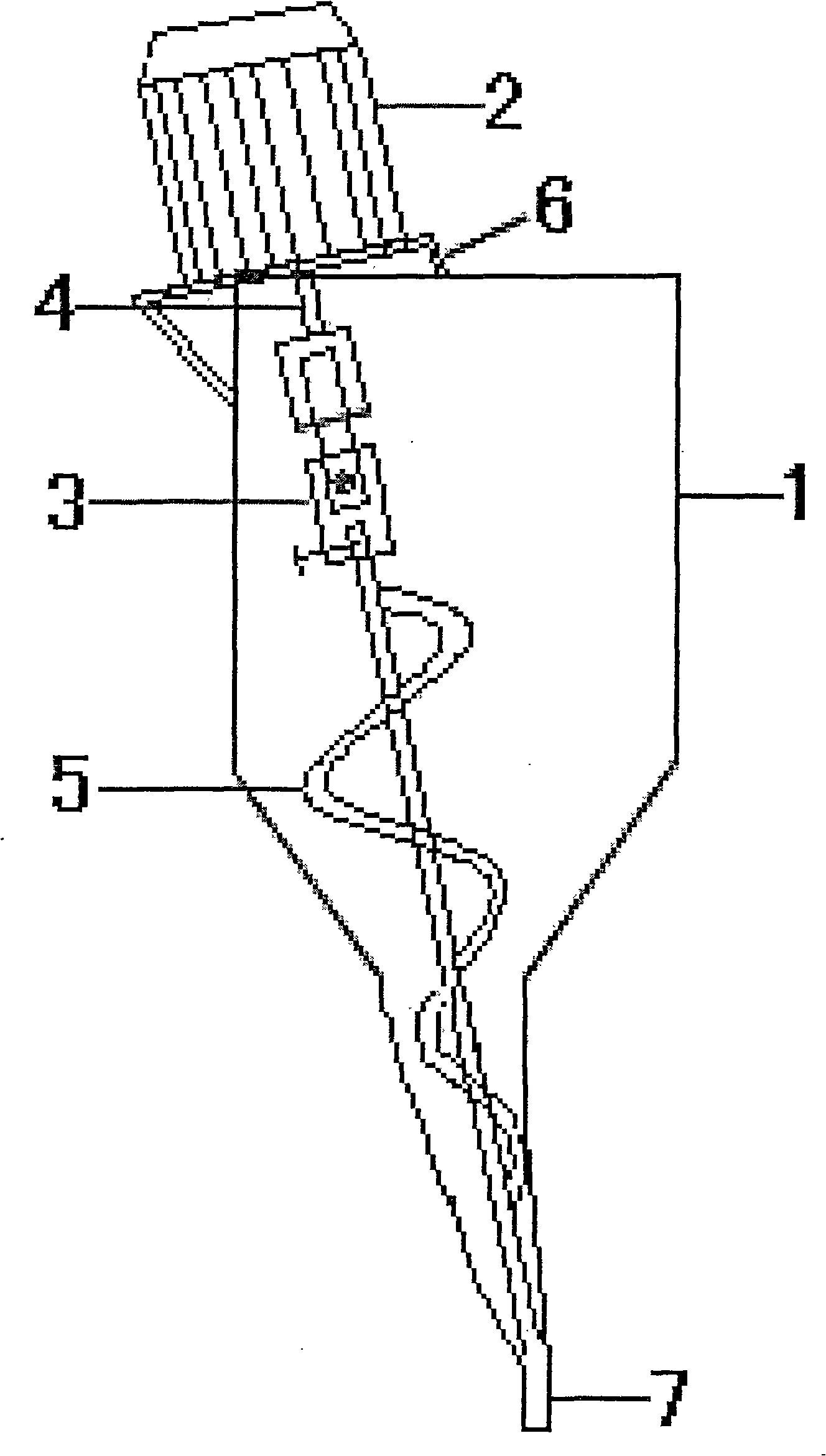
Method for preparing isosorbide mononitrate orally disintegrating tablets
A technology of isosorbide dinitrate and oral disintegrating tablets, which is applied in the directions of active ingredients of heterocyclic compounds, pill delivery, cardiovascular system diseases, etc., can solve the problems of inability to realize industrialized production, poor tablet compressibility, difference in tablet weight, etc. , to achieve the effect of cool and delicate taste, strong hardness, good compressibility, and small difference in tablet weight.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0029] Isosorbide Mononitrate 10g
[0030] Mannitol 53g
[0031] Microcrystalline Cellulose 22g
[0032] Low-substituted hydroxypropyl cellulose 3g
[0033] Croscarmellose Sodium 3g
[0034] Cross-linked polyvinylpyrrolidone 3g
[0035] Micronized silica gel 3g
[0037]
[0038] Makes 1000 pieces
[0039] Take isosorbide mononitrate and mannitol, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, cross-linked carmellose sodium, cross-linked polyvinylpyrrolidone, micropowder silica gel, and magnesium stearate as auxiliary materials, and press The prescription weight ratio is mixed and sieved, and the total mixed fine powder is placed in a powder feeder for a new tablet press machine, and compressed into tablets to produce isosorbide mononitrate orally disintegrating tablets.
example 2
[0041] Isosorbide Mononitrate 10g
[0042] Mannitol 30g
[0043] Microcrystalline Cellulose 60g
[0044] Low-substituted hydroxypropyl cellulose 10g
[0045] Croscarmellose Sodium 5g
[0046] Cross-linked polyvinylpyrrolidone 5g
[0047] Micronized silica gel 5g
[0049]
[0050] Makes 1000 pieces
[0051] Take isosorbide mononitrate and mannitol, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, cross-linked carmellose sodium, cross-linked polyvinylpyrrolidone, micropowder silica gel, and magnesium stearate as auxiliary materials, and press The prescription weight ratio is mixed and sieved, and the total mixed fine powder is placed in a powder feeder for a new tablet press machine, and compressed into tablets to produce isosorbide mononitrate orally disintegrating tablets.
example 3
[0053] Isosorbide Mononitrate 10g
[0054] Lactose 55g
[0055] Microcrystalline Cellulose 23g
[0056] Croscarmellose Sodium 3g
[0057] Sodium carboxymethyl starch 6g
[0058] Cross-linked polyvinylpyrrolidone 3g
[0059] Stevia 5g
[0060] Micronized silica gel 0.2g
[0061] Magnesium stearate 0.2g
[0062]
[0063] Makes 1000 pieces
[0064] Take isosorbide mononitrate and lactose, microcrystalline cellulose, cross-linked sodium carboxymethyl cellulose, sodium carboxymethyl starch, cross-linked polyvinylpyrrolidone, stevioside, micropowder silica gel, magnesium stearate and other auxiliary materials, and press The prescription weight ratio is mixed and sieved, and the total mixed fine powder is placed in a powder feeder for a new tablet press machine, and compressed into tablets to produce isosorbide mononitrate orally disintegrating tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com