Compound medicine effective ingredient pharmacokinetics and efficacy analysis method
A technology of active ingredients and analysis methods, applied in special data processing applications, instruments, electrical digital data processing, etc., can solve the cumbersome and difficult calculation operations of pharmacokinetics and pharmacodynamics parameters, mathematical methods and computer software are constantly updated, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
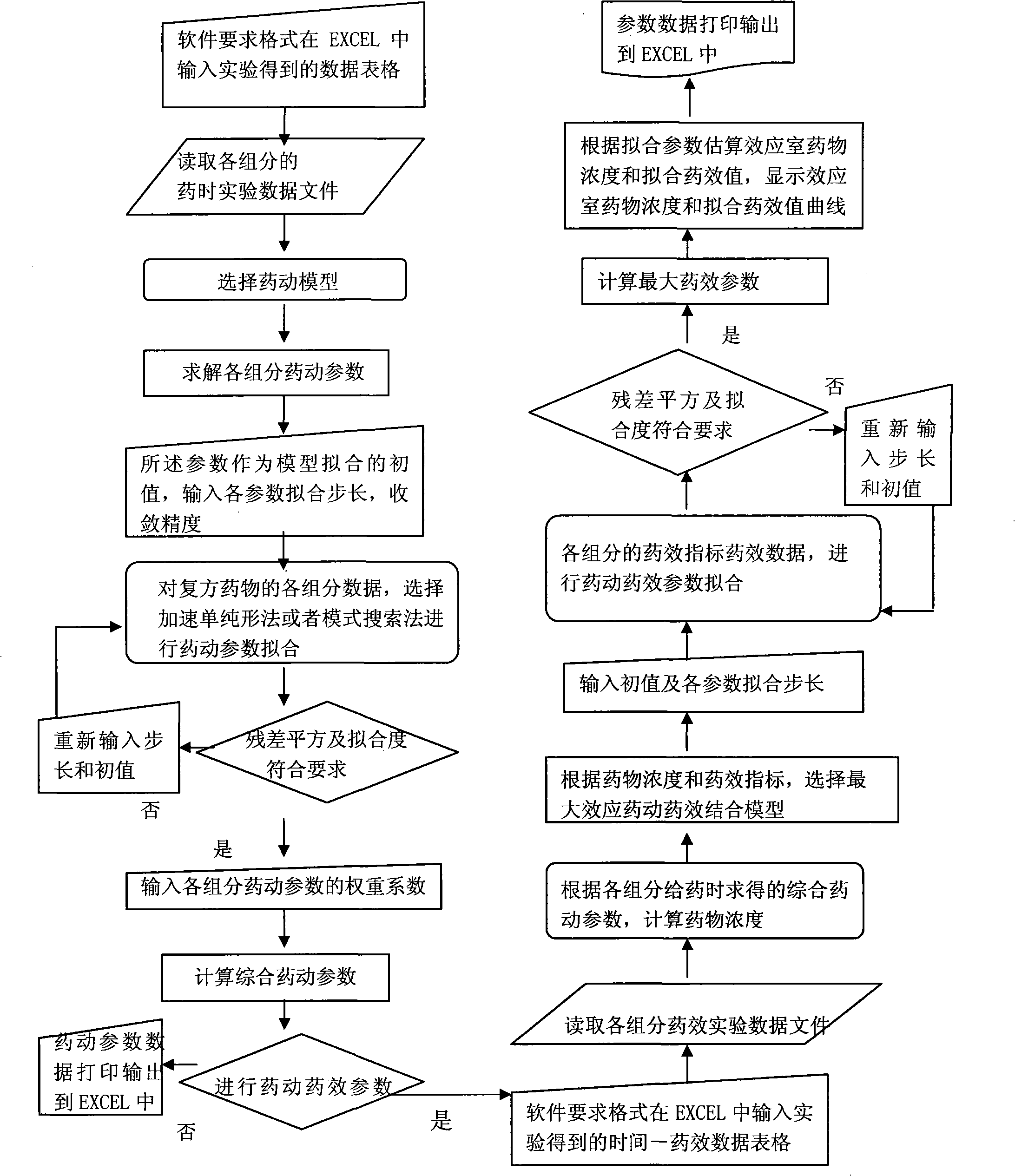
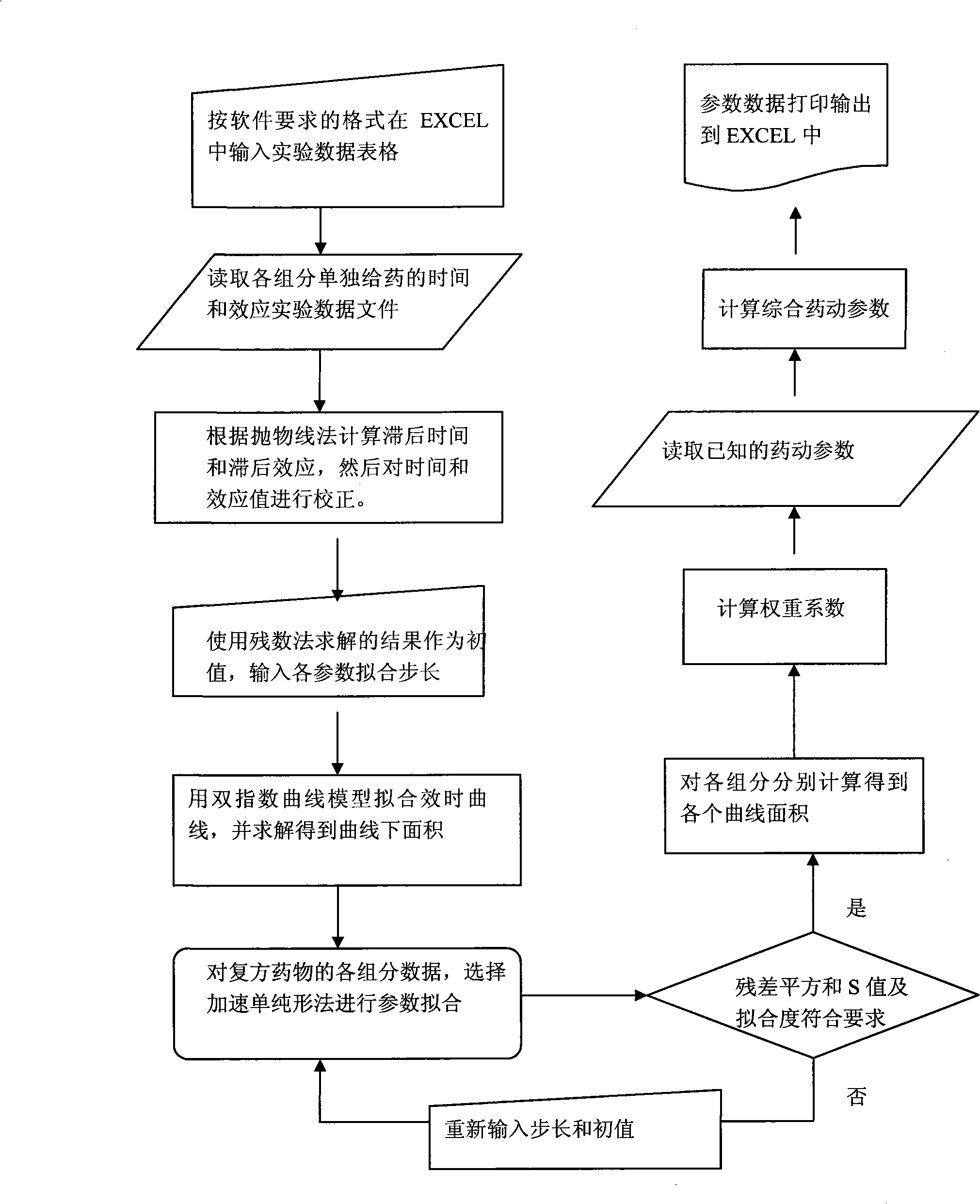
Image
Examples
Embodiment 1
[0030]The present invention is applied to the research on the pharmacokinetics and pharmacodynamics of the multi-component in vivo of a certain Chinese patent medicine.
[0031] 1) Obtain the drug time experimental data through experiments:
[0032] As shown in Table 1 below, when rats take multi-component Chinese patent medicines orally, the concentration of components 1 to 5 (concentration unit is ng / ml) changes according to the experimental data of drug time according to time.
[0033] Table 1 Data when rats take a certain Chinese patent medicine orally (concentration unit: ng / mL)
[0034]
[0035] 2) Obtain the time-effect experimental data of the time and effect of individual administration of each component through experiments:
[0036] Table 2 shows the effect on effect indicators I and II when rats take multi-component Chinese patent medicines orally, and the impact of single-component drugs on effect indicators I and II is shown in Table 3 and Table 4.
[0037] T...
Embodiment 2
[0077] The invention is applied to the study of the kinetics of single-component drugs in vivo.
[0078] The pharmacokinetic model is a two-chamber intravenous injection, and the fitting method is a pattern search method.
[0079] The experimental data and analysis results are shown in Table 12 and Table 13 below:
[0080] Table 12 Pharmacokinetic analysis results
[0081]
[0082] Note: *C: Concentration estimated value Pharmacokinetic parameter column element sequence (A, α, B, β) T
[0083] Table 13 Comparison of pharmacokinetic parameters and literature values
[0084]
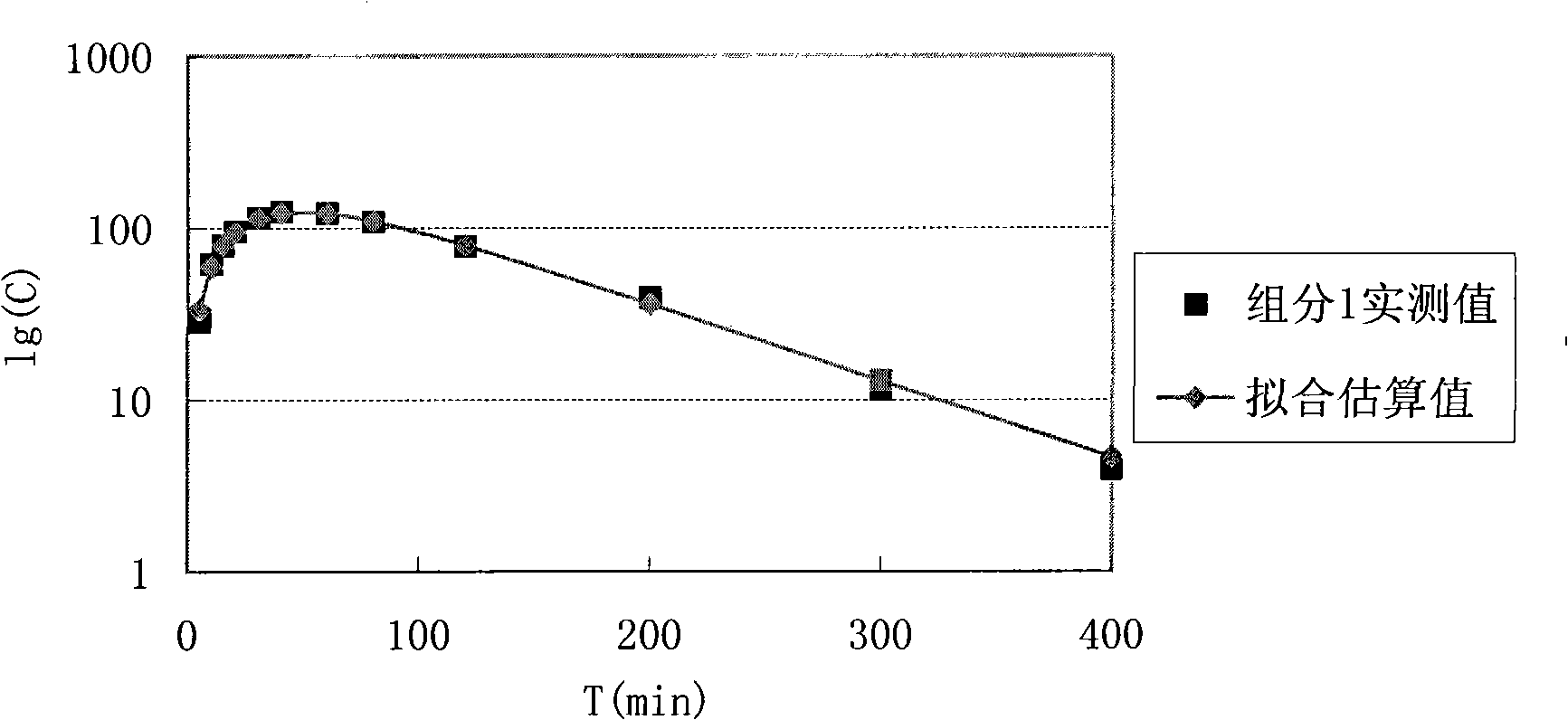
[0085] The fitting result is as Figure 10 shown.
[0086] The results show that the parameters calculated by this software are the same as the data that can be calculated by the current calculation software (reported in literature), and this software also increases the calculation of other parameters together.
Embodiment 3
[0088] The invention is applied to the research on the pharmacokinetic and pharmacodynamic combination model of the single-component medicine in vivo. The pharmacokinetic model is two-chamber intravenous injection, and the fitting method is the accelerated simplex method. The experimental data and analysis results are shown in Table 14 and Table 15.
[0089] Table 14 pharmacokinetic analysis results
[0090]
[0091] Note: *C: Estimated value of concentration; sequence of elements in pharmacokinetic parameter column (A, α, B, β) T
[0092] Table 15 Pharmacokinetic and pharmacodynamic combination analysis results
[0093]
[0094] Note: *E: experimental value of effect (urine output), E: estimated value of effect
[0095] In this embodiment, the time corresponding to the blood drug concentration fitting curve is as follows: Figure 11 Shown; Pharmacokinetic and pharmacodynamic analysis results are as follows Figure 12 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com