Process for preparation of meta-benzene dimethanamine by continuous hydrogenation reaction
A technology for m-xylylenediamine and isophthalonitrile, which is applied in the field of preparation of m-xylylenediamine, can solve the problem that the catalytic activity of the catalyst decreases rapidly, the disassembly, assembly, activation and regeneration are inconvenient, and the catalyst is easily worn, broken and lost. and other problems, to achieve the effects of inhibiting the formation of by-products, high hydrogen utilization rate, and not easy to wear and tear.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
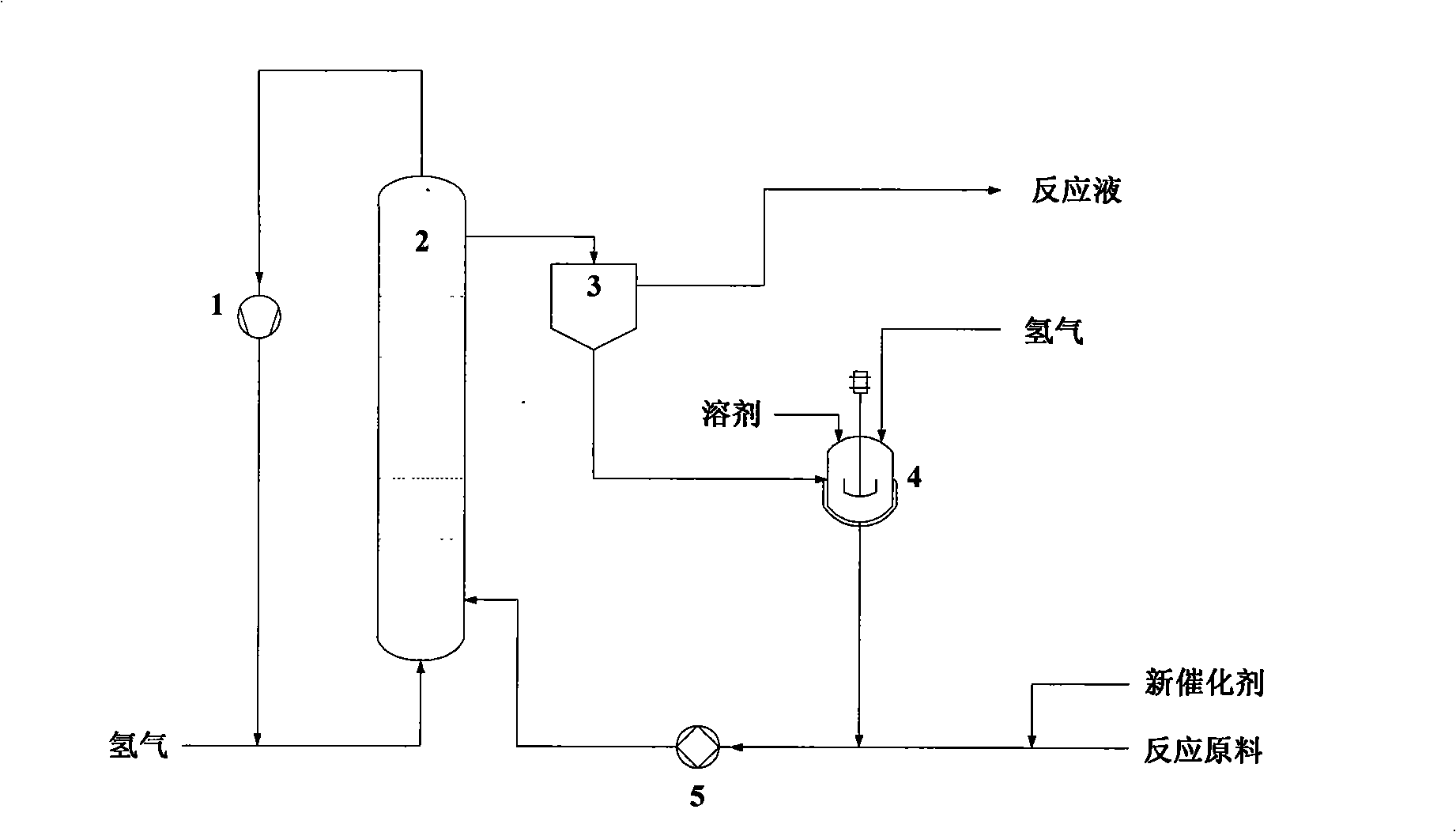
[0027] Example 1 Using the method of the present invention to prepare m-xylylenediamine by continuous hydrogenation of isophthalonitrile, the process flow is as follows figure 1 Shown.
[0028] Hydrogen, reaction raw materials (isophthalonitrile and solvent) and catalyst enter the slurry-bed reactor 2 from the bottom to perform continuous hydrogenation reaction of isophthalonitrile, and the reaction materials are separated in the head space of the reactor 2 to achieve gas-liquid separation. The tail gas discharged from the top of the reactor 2, that is, the unreacted hydrogen is pressurized by the compressor 1 and recycled; the liquid-solid mixture extracted from the upper part of the reactor 2 enters the liquid-solid separator 3 for liquid-solid separation, so that the solid catalyst and The reaction liquid is separated. The separated solid catalyst enters the stirred tank regenerator 4, adds an appropriate amount of solvent, and is washed and regenerated with the solvent in the...
Embodiment 2~5
[0032] In Examples 2 to 5, m-xylylenediamine was continuously prepared according to the process method of Example 1. The difference lies in the catalyst used in the hydrogenation reaction and the reaction temperature and pressure. When using Raney nickel catalyst, Raney nickel is a commercially available Raney nickel catalyst with a nickel content of 87.5% (weight); the weight ratio of Raney nickel catalyst to liquid is 0.08:1, and the hydrogen circulation volume is 120 standard cubic meters per hour. , The residence time of the material in the reactor is 2.25 hours. Raney nickel catalyst regeneration conditions are the same as in Example 1 except that the regeneration time is 3 hours.
[0033] The results of the reaction are shown in Table 1.
[0034] Table 1 Reaction results of continuous hydrogenation of isophthalonitrile to prepare metaxylylenediamine
[0035]
Embodiment 6
[0037] Example 6 The method described in the present invention was used to prepare m-xylylenediamine by continuous hydrogenation of isophthalonitrile. The catalyst regeneration adopts a slurry bed regenerator. The process flow is as follows figure 2 Shown.
[0038] Hydrogen, reaction raw materials (isophthalonitrile and solvent) and catalyst enter the slurry-bed reactor 2 from the bottom to perform continuous hydrogenation reaction of isophthalonitrile, and the reaction materials are separated in the head space of the reactor 2 to achieve gas-liquid separation. The tail gas discharged from the top of the reactor 2, that is, the unreacted hydrogen is pressurized by the compressor 1 and recycled; the liquid-solid mixture extracted from the upper part of the reactor 2 enters the liquid-solid separator 3 to separate the catalyst and the reaction liquid. The separated catalyst is added with a proper amount of solvent and enters the slurry bed regenerator 4, where it is washed and rege...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com