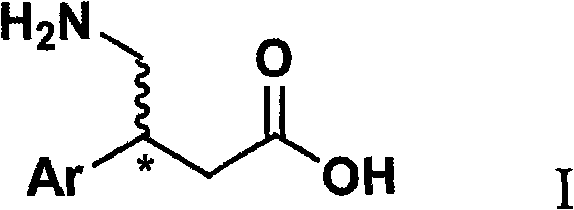
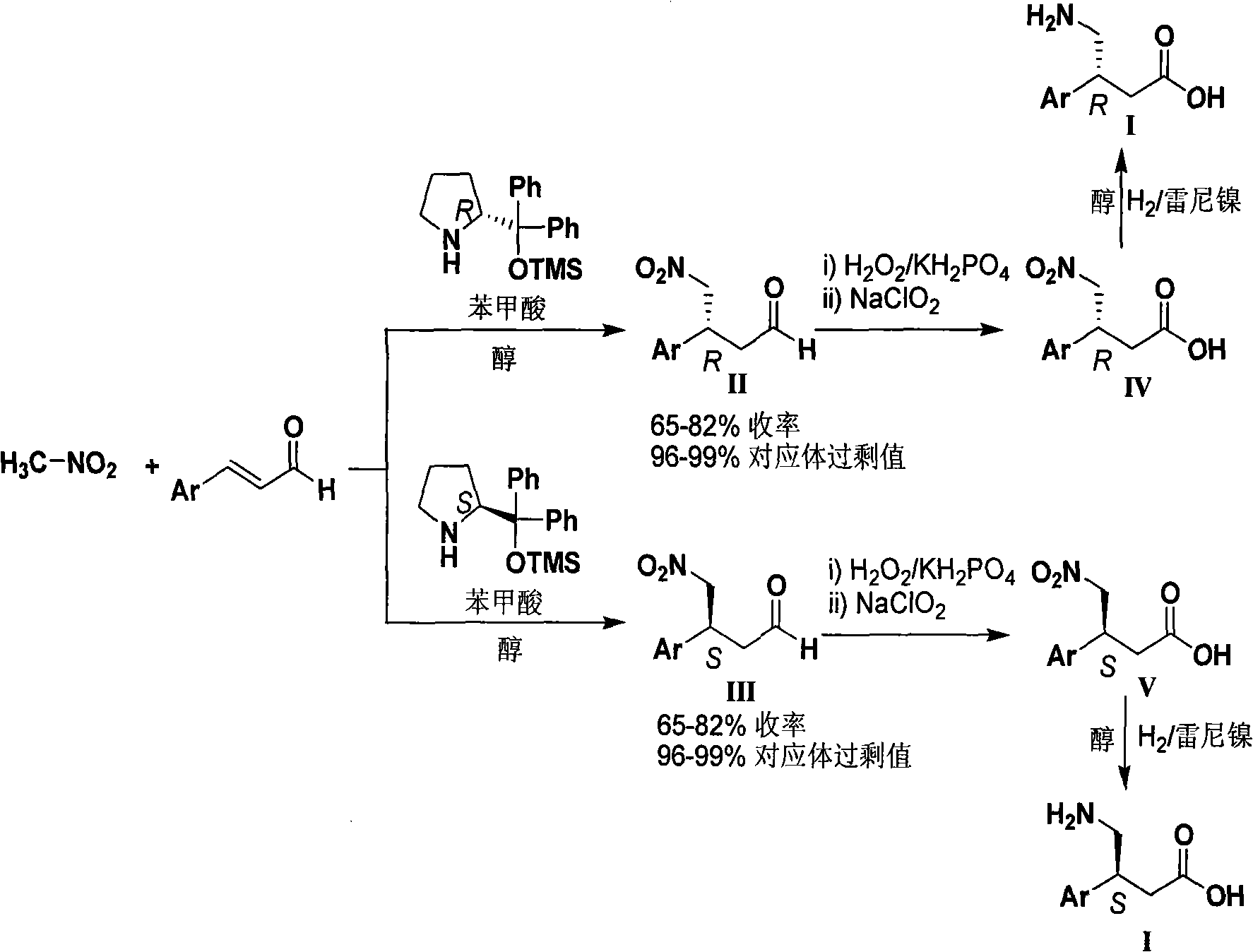
Novel method for synthesizing chiral beta-aryl-gamma-aminobutyric acid compounds
A technology of aminobutyric acid and a synthesis method, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry and other directions, can solve the problems of unobtainable starting materials, high preparation cost, complicated synthesis route and the like, and achieves reduction of synthesis cost. , the reaction conditions are mild and controllable, and the effect of shortening the reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (R)-3-(4-Chlorophenyl)-4-nitro-butyraldehyde (IIa)
[0029]
[0030] In 2 ml In ethanol, stirred and reacted at 0°C for 15 hours, the crude product was separated by column chromatography (eluent: ethyl acetate:petroleum ether=1:4) to obtain (R)-3-(4-chlorophenyl)- 4-nitrobutyraldehyde (IIa), yield (73%), 1 H NMR (500MHz, CDCl 3 ): 9.70(s, 1H), 7.32(d, 2H; J=8.5Hz), 7.18(d, 2H; J=8.0Hz), 4.57-4.69(m, 2H), 4.05-4.07(m, 1H) , 2.94 (d, 2H; J=6.5Hz); HPLC (Chiralpak AS-H, iPrOH / hexanes=30 / 70, flow rate=0.5mL / min, λ=210nm): t major =40.09min,t minor =32.08min, ee=96%; [α] D 25 =+11.5 (c=1.0in CHCl 3 ).
Embodiment 2
[0032] (S)-3-(4-Chlorophenyl)-4-nitro-butyraldehyde (IIIa)
[0033]
[0034] Replace (R)-diphenylpyrrolidine trimethylsilyl ether with (S)-diphenylpyrrolidine trimethylsilyl ether, and the rest of the required raw materials, reagents and preparation methods are the same as in Example 1 to obtain (S) -3-(4-chlorophenyl)-4-nitrobutyraldehyde (IIIa), yield (75%), 1 H NMR (500MHz, CDCl 3 ): 9.71(s, 1H), 7.32(d, 2H; J=8.5Hz), 7.18(d, 2H; J=8.0Hz), 4.57-4.69(m, 2H), 4.05-4.08(m, 1H) , 2.94(d, 2H; J=7.0Hz); 13 C NMR (125MHz, CDCl 3 ): δ198.2, 136.7, 129.4, 128.8, 79.1, 46.3, 37.3; HPLC (Chiralpak AS-H, i-PrOH / hexanes=30 / 70, flow rate=0.5mL / min, λ=210nm): t major =31.64min,t minor =43.79min, ee=97%; [α] D 25 =-11.7 (c=1.0 in CHCl 3 ).
Embodiment 3
[0036] (S)-3-(4-nitrophenyl)-4-nitro-butanal (IIIb)
[0037]
[0038] Replace (E)-3-(4-chlorophenyl)acrolein with (E)-3-(4-nitrophenyl)acrolein, and the rest of the required raw materials, reagents and preparation methods are the same as in Example 2 to obtain (S)-3-(4-nitrophenyl)-4-nitrobutanal (IIIb), yield 72%. 1 H NMR (500MHz, CDCl 3 ): 9.74(s, 1H), 8.22(d, 2H; J=8.5Hz), 7.45(d, 2H; J=8.5Hz), 4.65-4.79(m, 2H), 4.21-4.23(m, 1H) , 3.04(d, 2H; J=7.0Hz); 13 C NMR (125MHz, CDCl 3 ): δ197.5, 147.6, 145.7, 128.6, 124.3, 78.4, 46.1, 37.5; [α] D 25 =+7.2 (c=1.0in CHCl 3 ).ee=99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com