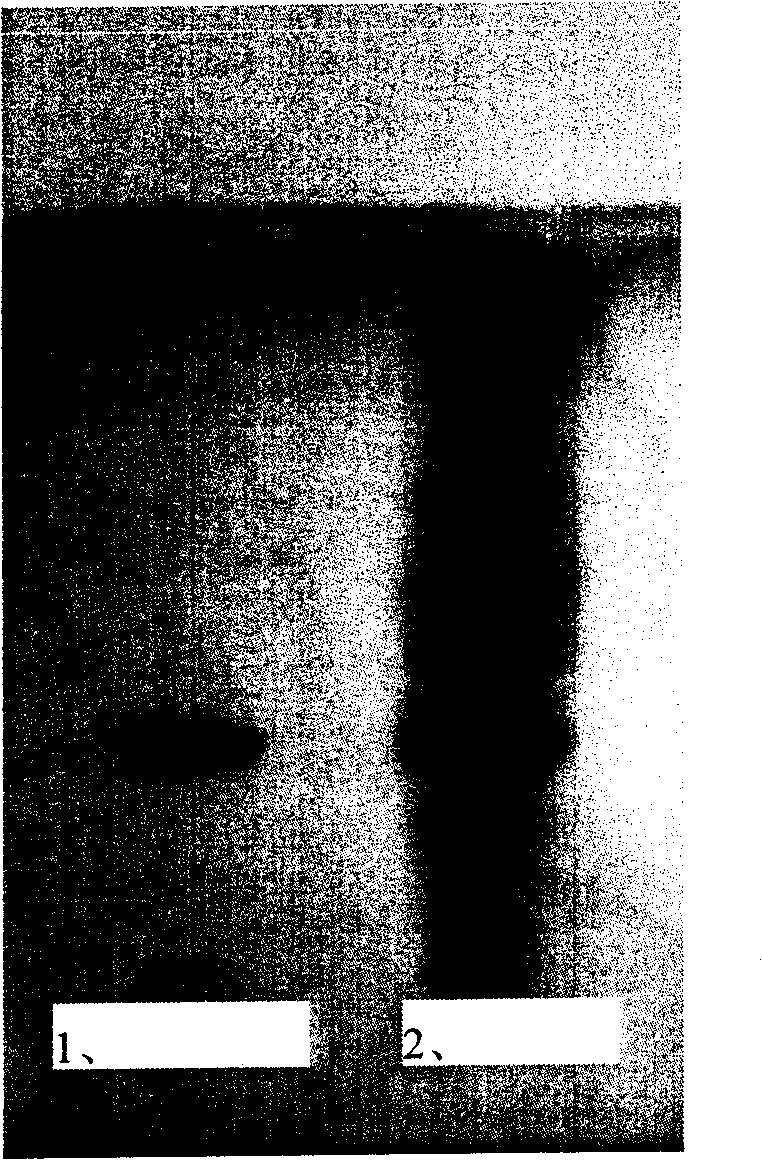Cordycepin polysaccharide buccal tablet and preparation method thereof
A technology of Cordyceps polysaccharides and tabletting, which is applied in the field of bioengineering, and can solve the problems of restricting the development process of polysaccharide products, single dosage form, limited throat moistening and local anti-inflammatory effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
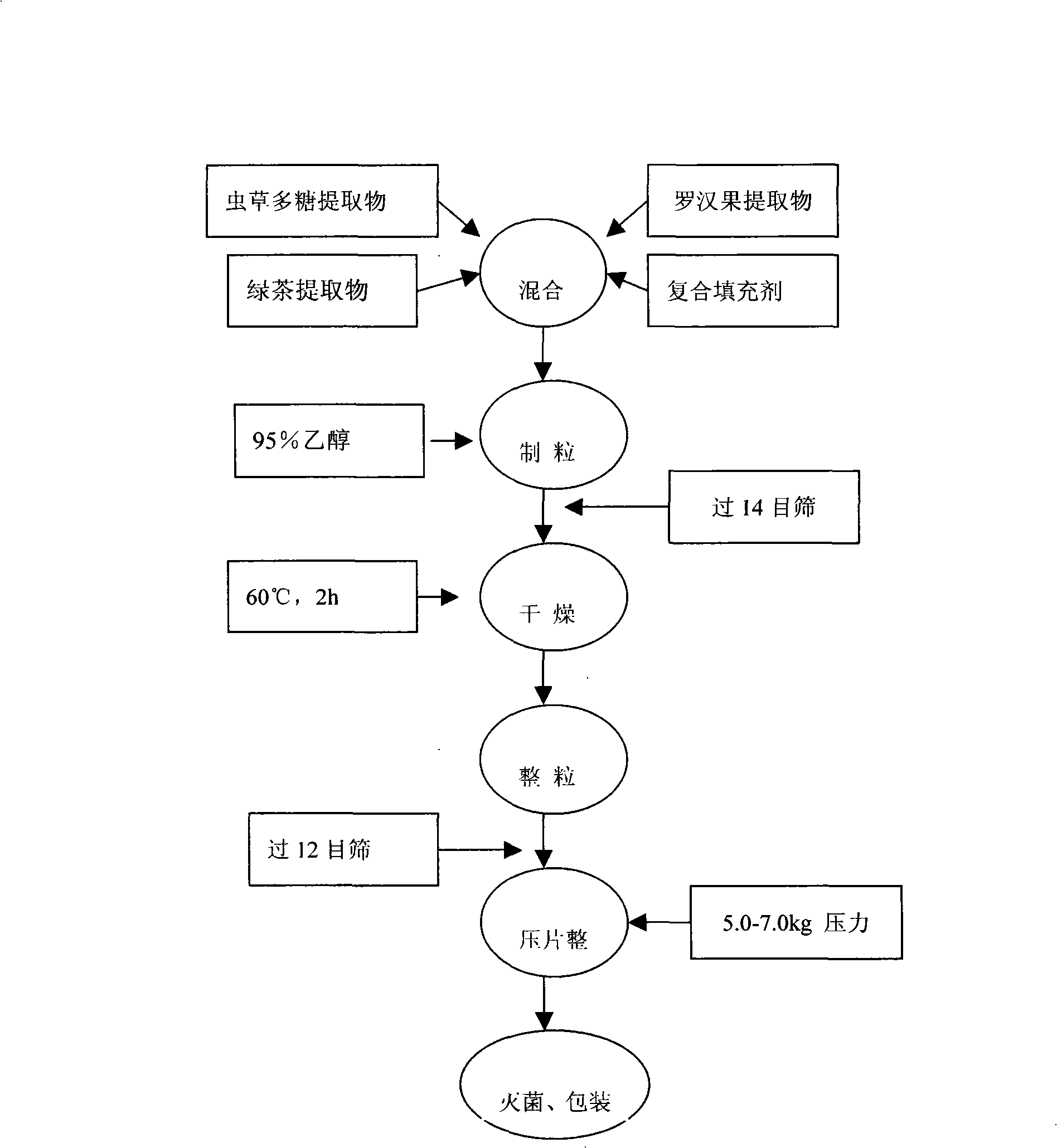
[0032] The preparation of embodiment 1 Cordyceps polysaccharide buccal tablet
[0033] Combination of raw and auxiliary materials ① Cordyceps polysaccharide extract: Cordyceps polysaccharide extract that has been crushed and passed through a 100-mesh sieve, the polysaccharide content is greater than 30%; ② Luo Han Guo extract: mogroside ≥ 80%; ③ green tea extract, tea polyphenols ≥ 80%; ④ composite filler: spray-dried mannitol 200SD is the main filler, with an additional amount of citric acid (or menthol), 10% polyvinylpyrrolidone (PVP) ethanol solution, magnesium stearate, mannitol and aspen Batian, etc., in line with national food hygiene requirements.
[0034] The various raw materials produced are combined into a mixture according to the following weight percentages for the next step of production.
[0035]
[0036] Production process Cordyceps polysaccharide extract, Luo Han Guo extract, green tea extract, + filler → (add 95% ethanol) granulate → (pass 14 mesh nylon s...
Embodiment 2
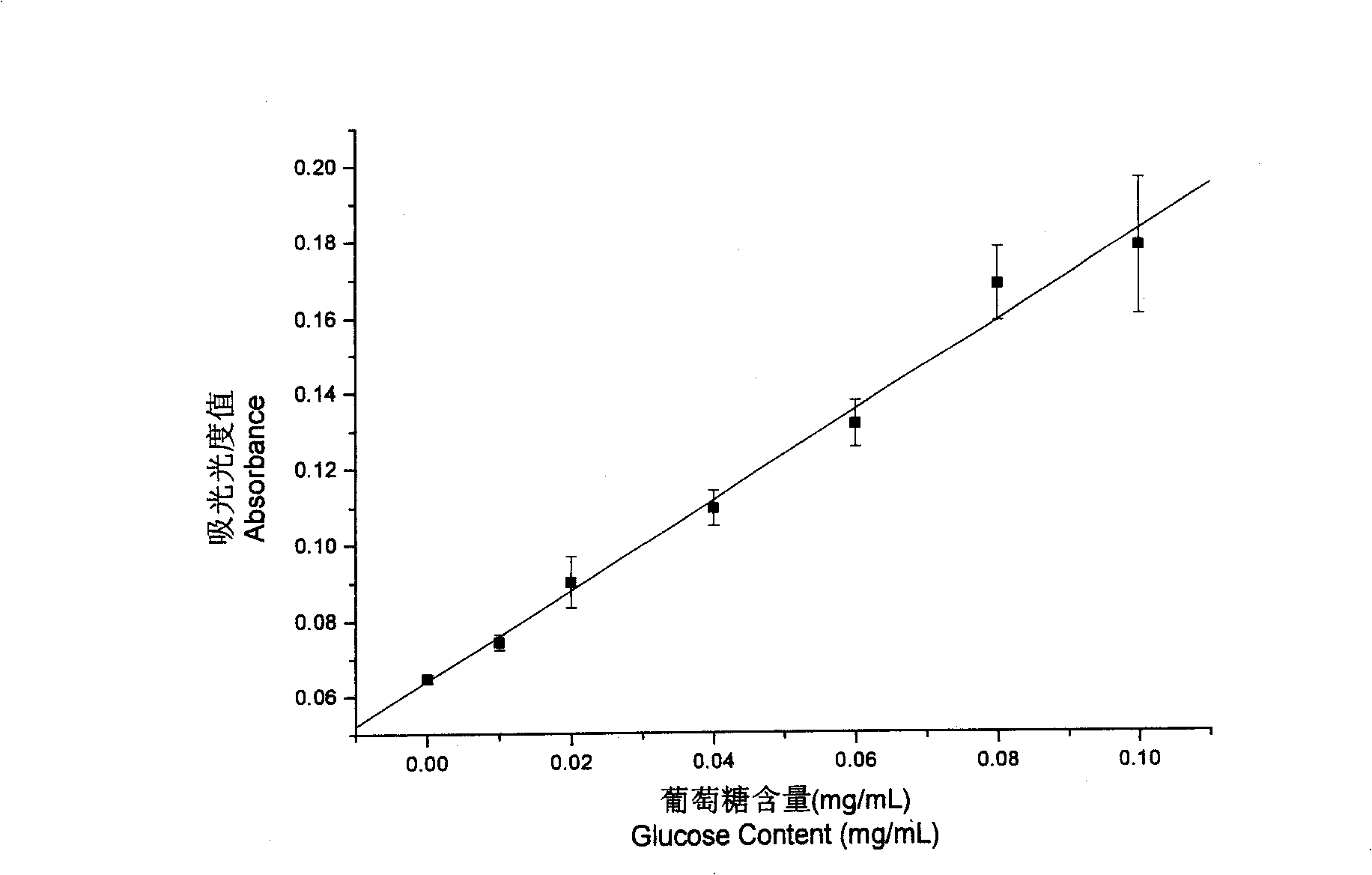
[0053] Detection of Cordyceps polysaccharide in embodiment 2 buccal tablets
[0054] The finished buccal tablets made from Combination 1, Combination 2 and Combination 3 were taken respectively to detect the polysaccharide content.
[0055] 1. Sample Processing
[0056] (1) Sample extraction: take 10 buccal tablets and grind them in a mortar, weigh 2.0 g of a solid sample that is uniformly mixed, add about 80 mL of water to a 100 mL volumetric flask, heat with boiling water for 2 hours, cool to room temperature and then supplement Add water to the mark, mix well, filter, discard the initial filtrate, and collect the remaining filtrate for precipitation of polysaccharides.
[0057] (2) Precipitate crude polysaccharide: Accurately draw 5.0mL of the final filtrate sample (or 5mL of liquid sample), put it in a 50mL centrifuge tube, add 20mL of absolute ethanol, mix for 5min, centrifuge at 3000r / min for 5min, and discard the supernatant liquid. Wash the residue with a few millil...
Embodiment 3
[0061] Example 3 TLC (thin-layer chromatography) qualitative identification of mogroside V in buccal tablets
[0062] The finished buccal tablets made from Combination 1, Combination 2 and Combination 3 were used for qualitative identification of Hanfructoside V.
[0063] (1) TLC conditions
[0064] Use 0.5% sodium carboxymethyl cellulose solution as the adhesive to pave the silica gel G plate with a thickness of 0.3mm and activate it at 105°C for 30min. Using n-butanol-ethanol-water (8:2:3) as the developer, develop upward with a span of 11 cm, develop color with 10% sulfuric acid ethanol, and bake at 105°C until the spots are clear.
[0065] (2) Preparation of reference substance solution
[0066] Accurately weigh 10.0 mg of Mogro side V reference substance, and dilute it in a 10 mL volumetric flask with distilled water to make a 1.0 mg / mL solution.
[0067] (3) preparation of test solution
[0068] Preparation of the test solution: Accurately weigh the compound Cordycep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com