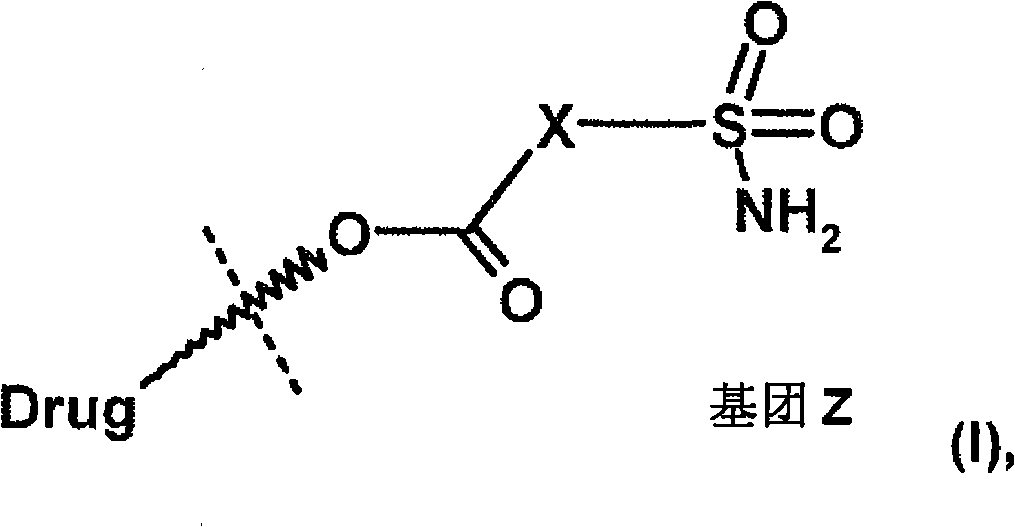
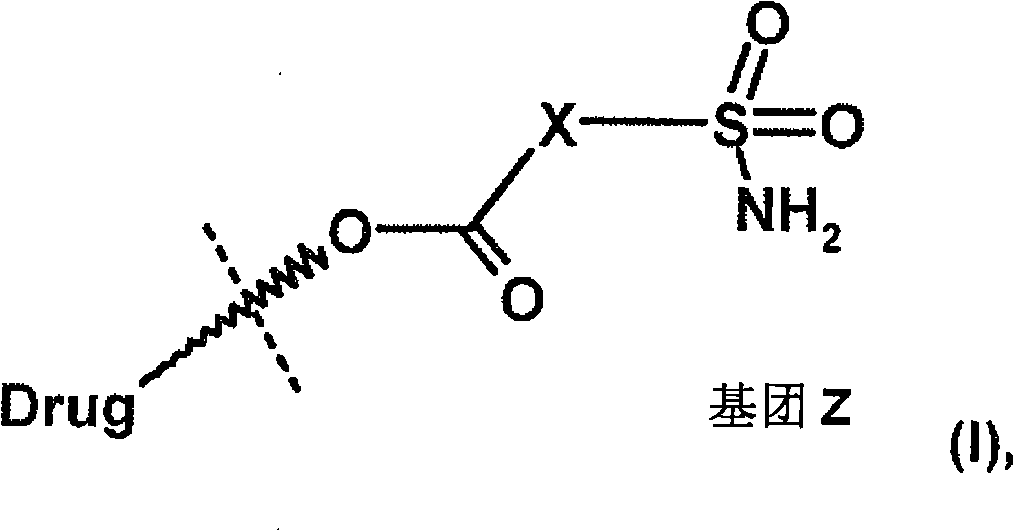
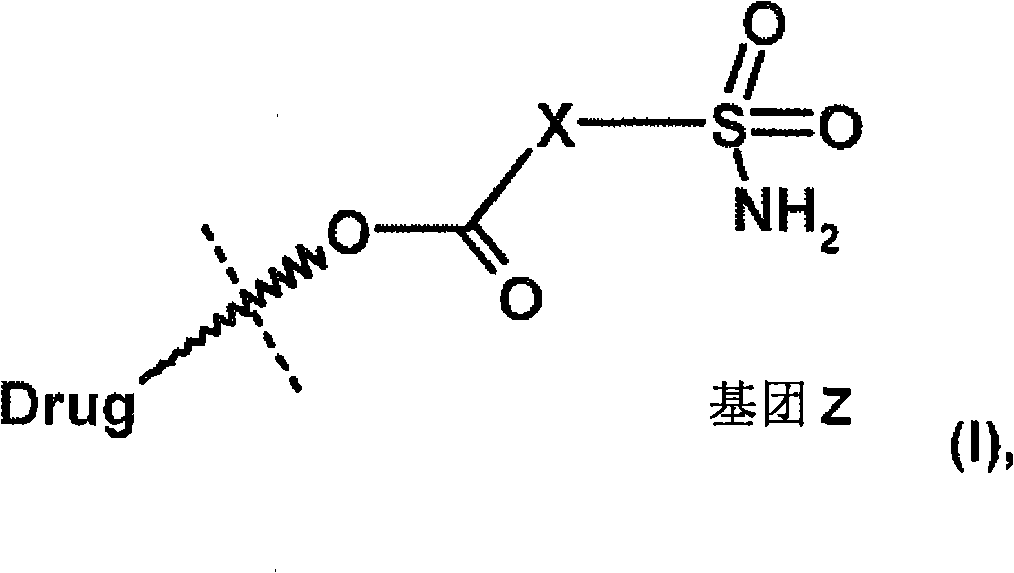
Heteroaromatic sulfonamide prodrugs
A technology of sulfonamide and heteroaromatic group is applied in the application field of preparing these prodrugs and preparing oral medicines, and can solve the problems of not providing active compounds, reducing metabolism and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: 3-tert-butyldimethylsilyloxyestra-1,3,5(10)-trien-17β-yl 6'-sulfamo acyl nicotinate
[0080] Under argon, 0.5 g of 3-tert-butyldimethylsilyloxyestra-1,3,5(10)-trien-17β-ol and 0.5 g of 6-sulfamoylnicotinic acid were dissolved in 7ml of pyridine. Then 0.1 g of p-Tos-OH was added and finally 0.5 g of DCC was added at 0°C. After 48 hours, the reaction mixture was stirred at room temperature. For work-up, 40 ml of water were added and the mixture was adjusted to pH~6 with 10% strength HCl. The precipitated material was filtered off, washed with water and dried. Purification by chromatography on silica gel affords 3-tert-butyldimethyl-silyloxyestra-1,3,5(10)-trien-17β-yl 6'-sulfamoyl-nicotinate .
[0081] 1 H-NMR (DMSO-d 6 ): 0.16(s, 6H, SiMe), 0.938(s, 9H, t-Bu), 0.944(s, 3H, 18-Me), 4.90(t, 1H, 17-H), 6.50-7.15(3m, 3H, CH Ar ), 7.69 (s, 2H, NH 2 ), 8.06, 8.55, 9.16 (3m, 3H, CH Py ).
Embodiment 2
[0082] Example 2: 3-Hydroxyestro-1,3,5(10)-trien-17β-yl 6'-sulfamoyl-nicotinate (1)
[0083] 300 mg of 3-tert-butyldimethylsilyloxyestra-1,3,5(10)-trien-17β-yl 6'-sulfamoylnicotinate was dissolved in 20 ml of THF. 250 mg of TBAF were added with stirring at room temperature. After 1 hour, 20 ml of water were added with stirring. This material was extracted with ethyl acetate. The organic phase was washed with saturated NaCl solution in MgSO 4 Drying, filtration, concentration and chromatographic purification on silica gel affords 3-hydroxyestra-1,3,5(10)-trien-17β-yl 6'-sulfamoyl nicotinate.
[0084] 1 H-NMR (DMSO-d 6 ): 0.94 (s, 3H, 18-Me), 4.90 (t, 1H, 17-H), 6.40-7.15 (3m, 3H, CH Ar ), 7.69 (s, 2H, NH 2 ); 8.06, 8.55 (2m, 2H, CH Py ), 9.02 (s, 1H, 3-OH), 9.17 (1s, 1H, CH Py ).
Embodiment 3
[0085] Example 3: 3-tert-butyldimethylsilyloxyestra-1,3,5(10)-trien-17β-yl 5'-sulfamo acyl nicotinate
[0086] Under argon, 0.55 g of 3-tert-butyldimethylsilyloxyest-1,3,5(10)-trien-17β-ol and 0.55 g of 5-sulfamoylnicotinic acid were dissolved in 7ml of pyridine. Then 0.12 g of p-Tos-OH were added and finally 0.55 g of DCC were added at 0°C. After 48 hours, the reaction mixture was stirred at room temperature. For work-up, 40 ml of water were added and the mixture was adjusted to pH~6 with 10% strength HCl. The precipitated material was filtered off, washed with water and dried. Chromatographic purification on silica gel yields 3-tert-butyldimethyl-silyloxyestra-1,3,5(10)-trien-17β-yl 5'-sulfamoyl-nicotinate .
[0087] 1 H-NMR (DMSO-d 6 ): 0.16(s, 6H, SiMe), 0.94(s, 9H, t-Bu), 0.95(s, 3H, 18-Me), 4.92(t, 1H, 17-H), 6.5-7.2(3m, 3H, CH Ar ), 7.79 (s, 2H, NH 2 ), 8.6-9.3 (3m, 3H, CH Py ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com