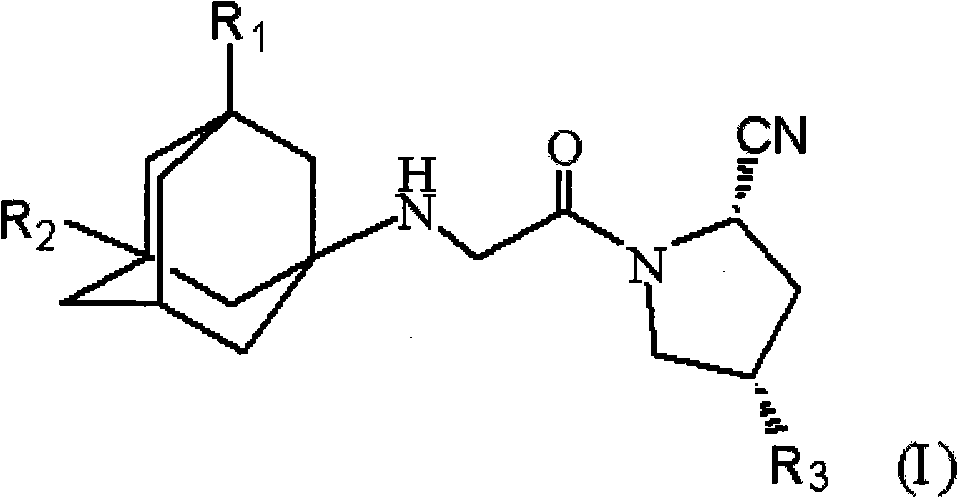
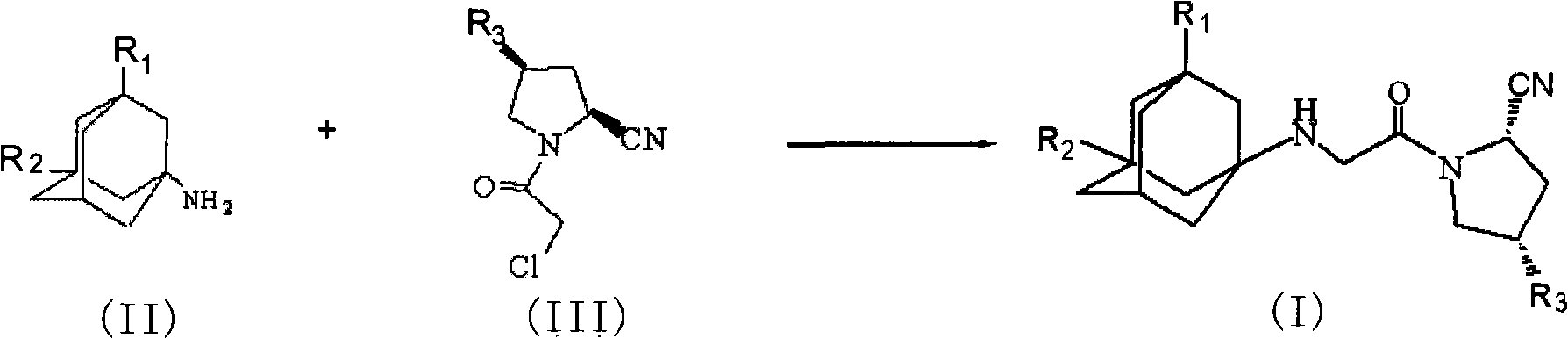Novel dipeptidyl peptidase restrainer, synthesizing process and uses thereof
A technology of use and solvate, applied in the field of a new type of dipeptidyl peptidase inhibitor, synthesis and use, capable of solving problems such as unsatisfactory inhibitory properties of dipeptidyl peptidase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] In the preparation method of the present invention, each reaction is usually carried out in an inert solvent at 0°C to solvent reflux temperature (preferably room temperature to 80°C). The reaction time is usually 0.1 to 60 hours, preferably 0.5 to 48 hours.
[0048] In a preferred example, the compound of formula (I) of the present invention can be prepared according to the following route I:
[0049] Route I
[0050]
[0051] Among them, a: SOCl 2 , CCl 4 , b: 1-chloroacetyl-2-cyanopyrrole, K 2 CO 3 , THF
[0052] Step 1.3-Hydroxy-1-adamantanamine hydrochloride 1 [Synthetic Communications; 1988, 18 (16): 1975-1978], in an aprotic solvent, reacted with a chlorination reagent in the presence of a base to obtain 3 in high yield -Chloro-1-adamantanamine.
[0053] Suitable solvents for the above reaction are carbon tetrachloride, dichloromethane, benzene, toluene, dimethylformamide and chloroform, and suitable bases are triethylamine and pyridine. The chlorinati...
Embodiment 1
[0139] Example 1: (2S)-1-[(3-fluoro-1-adamantyl)aminoacetyl]-2-cyanopyrrolidine (YF-1)
[0140] At room temperature, add 3-fluoro-1-adamantanamine hydrochloride (245mg, 1.45mmol), potassium carbonate (500mg, 3.6mmol) and tetrahydrofuran (15ml) into the reaction flask together, start stirring, dropwise add (2S )-1-Chloroacetyl-2-cyanopyrrole (100mg, 0.58mmol) in tetrahydrofuran, after the dropwise addition, sodium iodide (0.1g) was added. The reaction mixture was stirred at room temperature for 15 h, and was analyzed by TLC (CH 2 Cl 2 / MeOH=10:1) to monitor the reaction. After the reaction was complete, it was cooled, and the potassium carbonate solid was filtered off, and the filtrate was evaporated to dryness under reduced pressure, and directly carried out column chromatography, (developing agent: CH 2 Cl 2 / MeOH=25:1) to obtain a yellow viscous substance. A saturated solution of hydrogen chloride in diethyl ether was added to the yellow viscous substance to generate hy...
Embodiment 2
[0142] Embodiment 2: (2S)-1-[(3-chloro-1-adamantyl)aminoacetyl]-2-cyanopyrrolidine (YF-2) 1.3-chloro-1-adamantanamine (compound 2 )
[0143] Dissolve 3-hydroxyadamantamine (500mg, 3mmol) in carbon tetrachloride (20ml), add thionyl chloride (0.5ml, 7.5mmol), stir and reflux for 22h, spin out the solvent to obtain a viscous substance (650mg , 100%). directly into the next reaction.
[0144] 1 H NMR (400MHz, CDCl 3 ) δ 1.46-1.56 (m, 8H), 1.97-2.07 (m, 6H), 2.25 (s, 2H).
[0145] 2. (2S)-1-[(3-Chloro-1-adamantyl)aminoacetyl]-2-cyanopyrrolidine (YF-2)
[0146] Compound 2 (555 mg, 3.0 mmol) and (2S)-1-chloroacetyl-2-cyanopyrrole (172 mg, 1 mmol) were reacted and treated according to the method of Example 1 to obtain a white solid (54 g, 5.6%).
[0147] Mp: 134-138°C. 1 H NMR (400MHz, CDCl 3 ) δ 1.49-2.29 (m, 19H), 3.35-3.59 (m, 4H), 4.71-4.73 (dd, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com