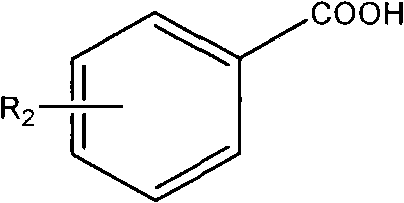
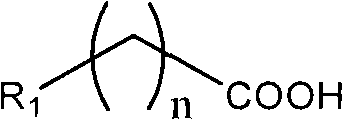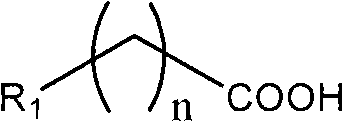Preparation method for converting organic carboxylic acid into organic aldehyde
A technology of organic carboxylic acid and organic aldehyde, which is applied in the field of preparation of organic carboxylic acid into organic aldehyde, can solve the problems of low yield in the reaction process, industrial application limitations, and difficult handling, and achieve simple process, rapid reaction, tractable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The preparation of n-octanal: 1) In a dry three-necked flask of 250ml, add n-octanoic acid 9.5ml (60mmol) and 100ml anhydrous ether, stir vigorously, add borane-dimethyl sulfide 6.12ml (60mmol) dropwise under normal temperature , Refluxed for 1 hour after the dropwise addition. Stop the reaction, remove anhydrous ether, and then add 50ml of acetone to obtain an acetone solution of the oxyboryl compound. 2) Add 0.01 g of TEMPO and 6.4 g of sodium carbonate to 50 ml of the acetone solution of the oxyboryl compound, and at 25° C., add dropwise 50 mL of the acetone solution of TCCA containing 5.1 g of TCCA (22 mmol) and stir; React for another 30 min, stop the reaction. Add water 30ml NH 4 The saturated aqueous solution of Cl was extracted three times with 150 ml of dichloromethane, dried, and the solvent was removed to obtain 7.4 g of the product with a yield of 96%.
Embodiment 2
[0020] The difference from Example 1 is that the preparation of 9-acyloxy n-nonanoic acid methyl ester: 12.1 g (60 mmol) of 9-methoxy-9-acyloxy n-nonanoic acid is dissolved in tetrahydrofuran solution, dropwise at room temperature Add 6.12ml (60mmol) of borane-dimethylsulfide, and then add 150ml of dichloromethane to obtain a dichloromethane solution of the oxyboryl compound. Then add 0.05g TEMPO and 5.8g pyridine, add dropwise 50ML acetone solution containing 5.6g TCCA at 0°C and stir for 60 minutes after the addition is complete, then stop the reaction. That is, the target product was obtained with a yield of 98%.
Embodiment 3
[0022] The difference from Example 1 is that the preparation of p-chlorobenzaldehyde: 9.4g (60mmol) of p-chlorobenzoic acid is dissolved in anhydrous ether solution, and 6.6ml (65mmol) borane-dimethyl sulfide is added dropwise at room temperature, Then add 50ml of acetone to obtain an acetone solution of the oxyboryl compound. Then add 0.02g TEMPO and 7.0g picoline, add dropwise 100ML acetone solution containing 6.0g TCCA at 5°C and stir, react for another 60min after the dropwise addition, and stop the reaction. That is, the target product was obtained with a yield of 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com