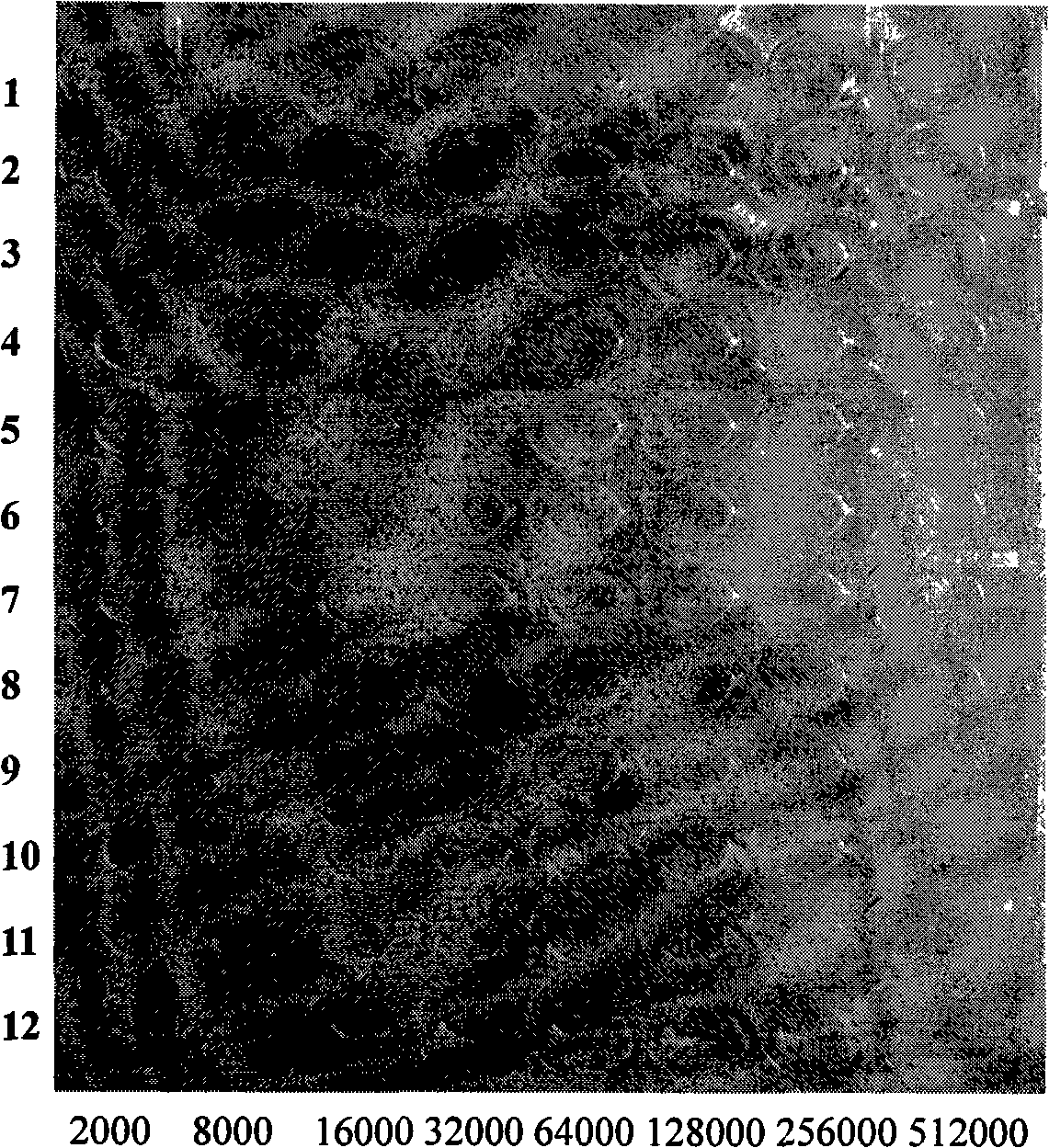
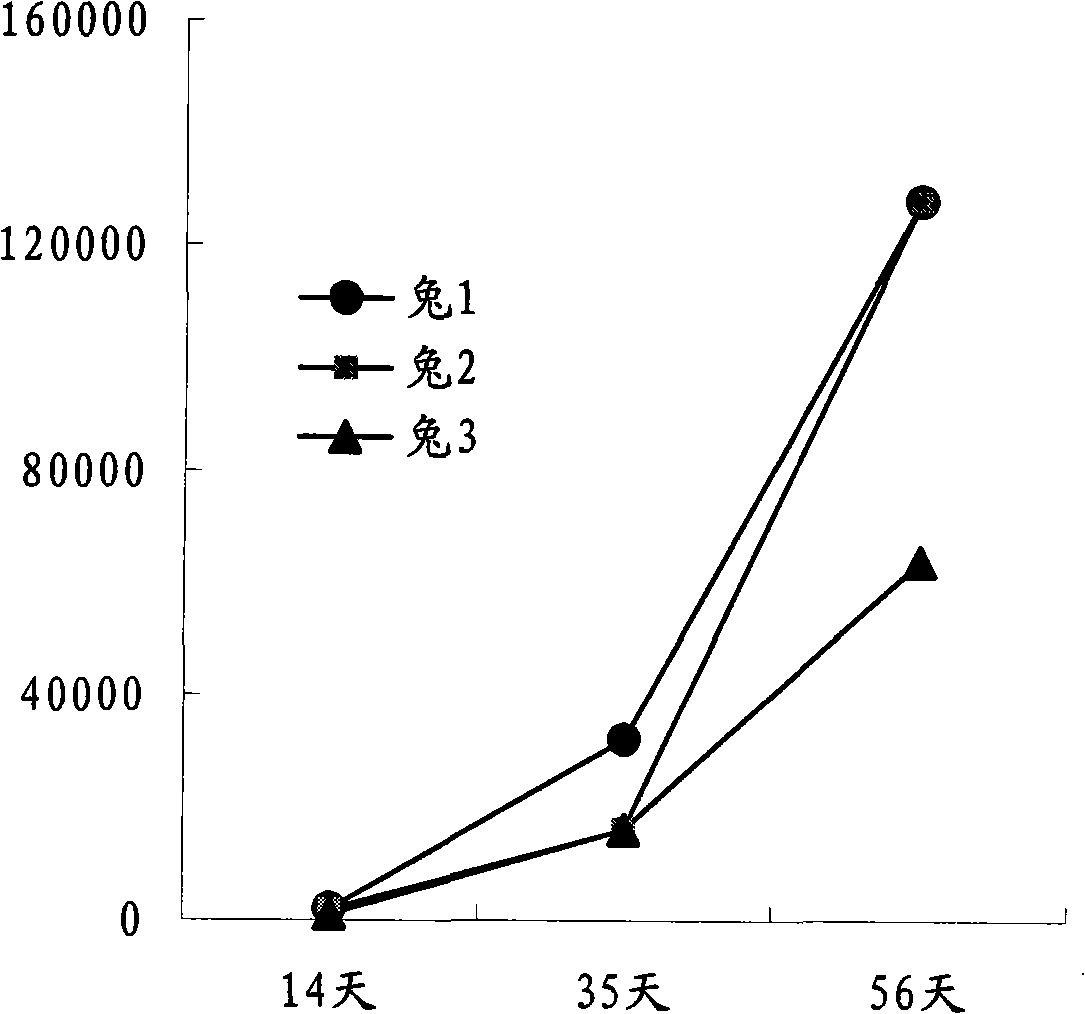
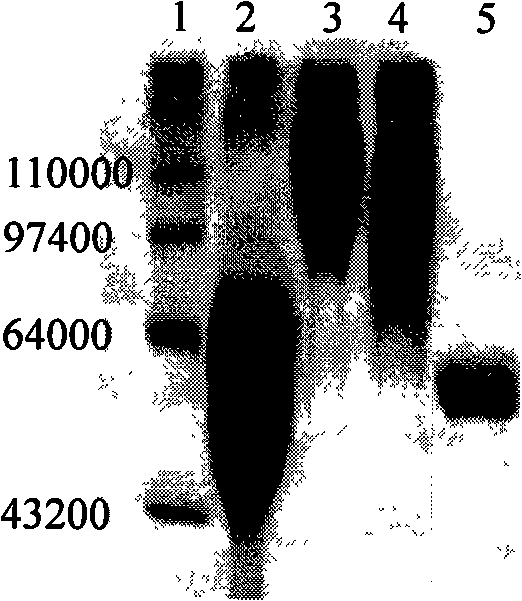Tumour treatment vaccine CTP37CRM197 immunogen, preparation method and application thereof
A technology of CTP37CRM197 and CRM197, applied in the field of medicine, can solve the problems of not reaching the theoretical cross-linking rate and the difference in the cross-linking rate of sugars
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] 1. Materials used
[0104] 1.1 Main reagents
[0105] Monohydrate cysteine hydrochloride (Cysteine Hydrochloride Monohydrate), sulfhydryl group determination reagent Ellman reagent, cross-linking agent EMCS: product of Sigma company; Fluorescamine: product of Sigma company;
[0106] Others are domestic reagents.
[0107] 1.2 Main instruments and equipment
[0108] TGL-16 high-speed desktop centrifuge: product of Shanghai No. 6 Medical Device Factory.
[0109] Micropipettes: products of Eppendof, Germany.
[0110] HD95-1 protein detector and 3057 recorder: (Shanghai Kanghua Biochemical Instrument Factory). Peristaltic pump: Lange pump, YZ1515, Baoding Lange Constant Flow Pump Co., Ltd. BHW-IV electric heating constant temperature water temperature box: Beijing Medical Equipment Factory. J2-MC high-speed low-temperature centrifuge: Beckman company products. Ice machine (GRANT), 1-15 centrifuge (Sigma), Spectrumlad54 UV-Vis spectrophotometer (Shanghai Prism Tech...
Embodiment 2
[0137] 1. In vitro tumor inhibition experiment
[0138] 1.1 Anti-CTP37CRM197 antibody prepared by the present invention inhibits the growth of tumor cells
[0139] Add the antibody prepared in Example 1 to well-growing tumor cells, and measure the cell viability of different treatment groups (adding CTP37 group, adding antibody group, blank control group) by MTT method to determine the inhibitory effect of the antibody on tumor cells . The selected tumor cells are gastric cancer cell MKN45 and colorectal cancer cell SW480.
[0140] Tumor cell lines were cultured in vitro, and sterile CTP37 and CTP37 antibodies were added to 10% calf serum RPMI1640 culture medium, and divided into three groups: blank control group, CTP37 group (25ug / m1) and antibody group (30ug / m1).
[0141] Determination of cell viability: use thiazolium blue (MTT) colorimetric assay to measure cell viability, take 100ul of cell-containing culture fluid (cell concentration is 10 5 / m1), inoculated in 96-wel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com