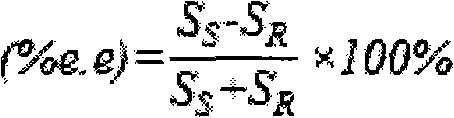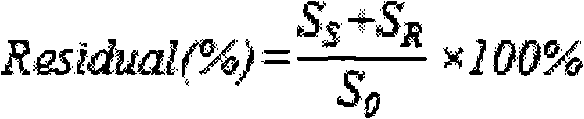Method for preparing (S)-benzoglycol by microorganism unsymmetrical split
A kind of phenylethylene glycol, microorganism technology, applied in biological field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 , strain culture
[0034] Prepare seed medium and fermentation medium according to the following formulas:
[0035] DSM2003 strain seed medium: take 8.0g sorbitol, 2.0g yeast extract, 0.1g KH 2 PO 4 , 0.05g MgSO 4 ·7H 2 O, pH6.0, add distilled water to make 100ml culture medium.
[0036] DSM2003 strain fermentation medium: take 8.0g sorbitol, 2.0g yeast extract, 0.1g KH 2 PO 4 , 0.05g MgSO 4 ·7H 2 O, pH 6.0 was added to distilled water to make 100ml culture medium.
[0037] Take the DSM2003 strain of Gluconobacter oxydans (Gluconobacter oxydans), add it to the seed medium, and culture it with shaking at 200 rpm for 24 hours at 28°C to obtain the seed culture solution, and then add the seed culture solution to the fermentation culture at an inoculum size of 10%. medium, at 25-30°C, 100-300rpm, cultivated for 24 hours, and harvested the fermentation broth.
[0038] The fermentation broth was centrifuged to collect the bacterial cell precipitate, and t...
Embodiment 2
[0039] Example 2 , the influence of reaction time on preparation (S)-phenylethylene glycol
[0040] In the 40ml 0.1mol / L phosphate buffer solution that racemic phenylethylene glycol (substrate) concentration is 10g / L, add the wet thalline of the DSM2003 that 0.3g embodiment 1 obtains, carry out selective oxidation reaction, between reactor , adjust the pH to 5.5 intermittently, and the reaction temperature is 28°C.
[0041] As shown in Table 1, samples were taken at regular intervals, and the supernatant was collected after centrifugation of the sample, extracted with an equal volume of ether, and the organic phase was collected to detect (R)-phenylethylene glycol, (S)-phenylethylene glycol and (R, S)-phenylethylene glycol content, calculate the optical purity and the productive rate of (S)-phenylethylene glycol, the results are shown in Table 1.
[0042] Table 1, the influence of reaction time on preparation (S)-phenylethylene glycol
[0043] Reaction time
[0...
Embodiment 3
[0045] Example 3 , the influence of substrate concentration on the preparation of (S)-phenylethylene glycol
[0046] As shown in table 2, in the 10ml 0.1mol / L phosphate buffer solution of different racemic phenylethylene glycol (substrate) concentrations, add the wet thalline of the DSM2003 that a certain amount of embodiment 1 obtains, carry out selective oxidation reaction , the reaction maintained at pH 6.0, and the reaction temperature was 28°C.
[0047] Stop the reaction after 36 hours, centrifuge the reaction solution, collect the supernatant, extract with an equal volume of ether, collect the organic phase, and detect (R)-phenylethylene glycol, (S)-phenylethylene glycol and (R, S) -Phenylethylene glycol content, calculate the optical purity and productive rate of (S)-phenylethylene glycol, the results are shown in table 2.
[0048] Table 2, the influence of substrate concentration on preparation (S)-phenylethylene glycol
[0049] Substrate concentration
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com