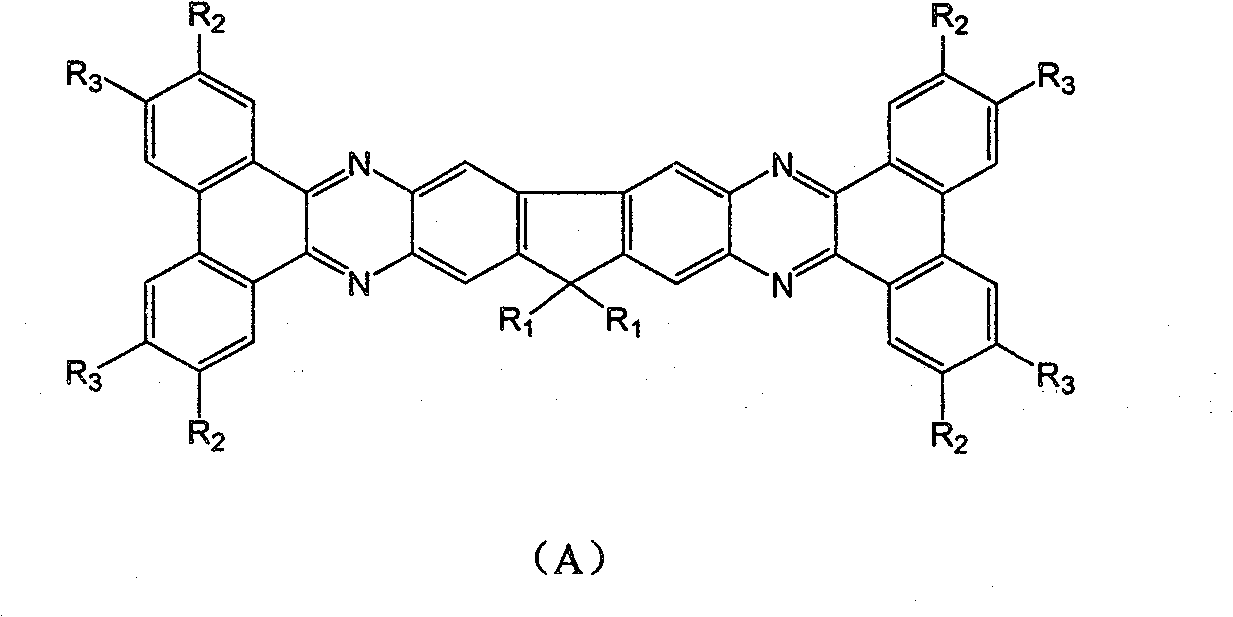
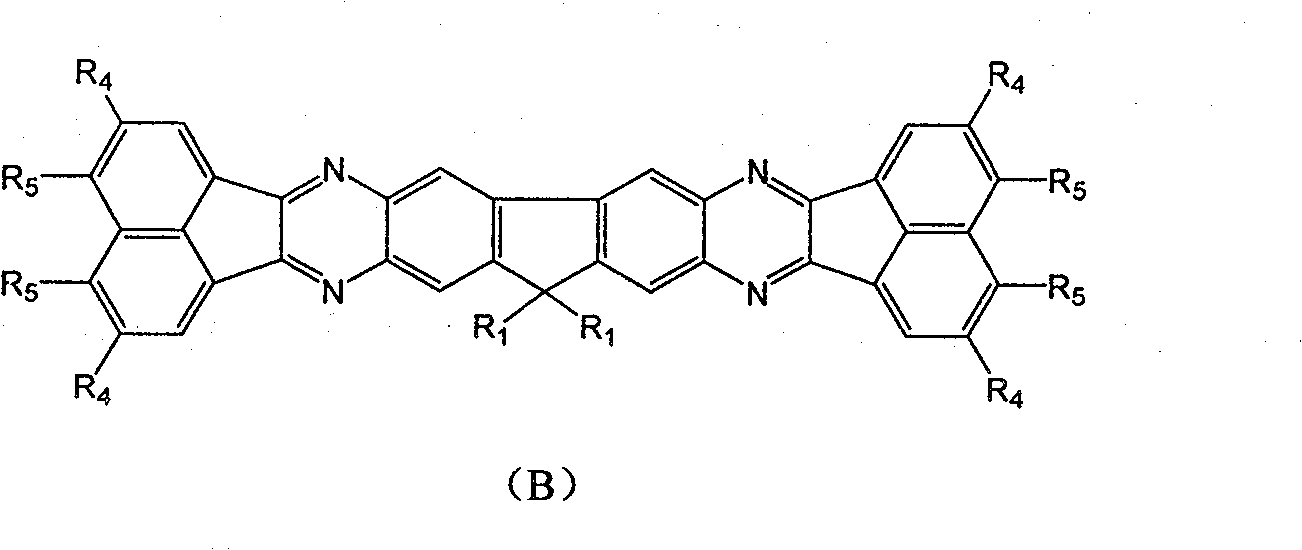
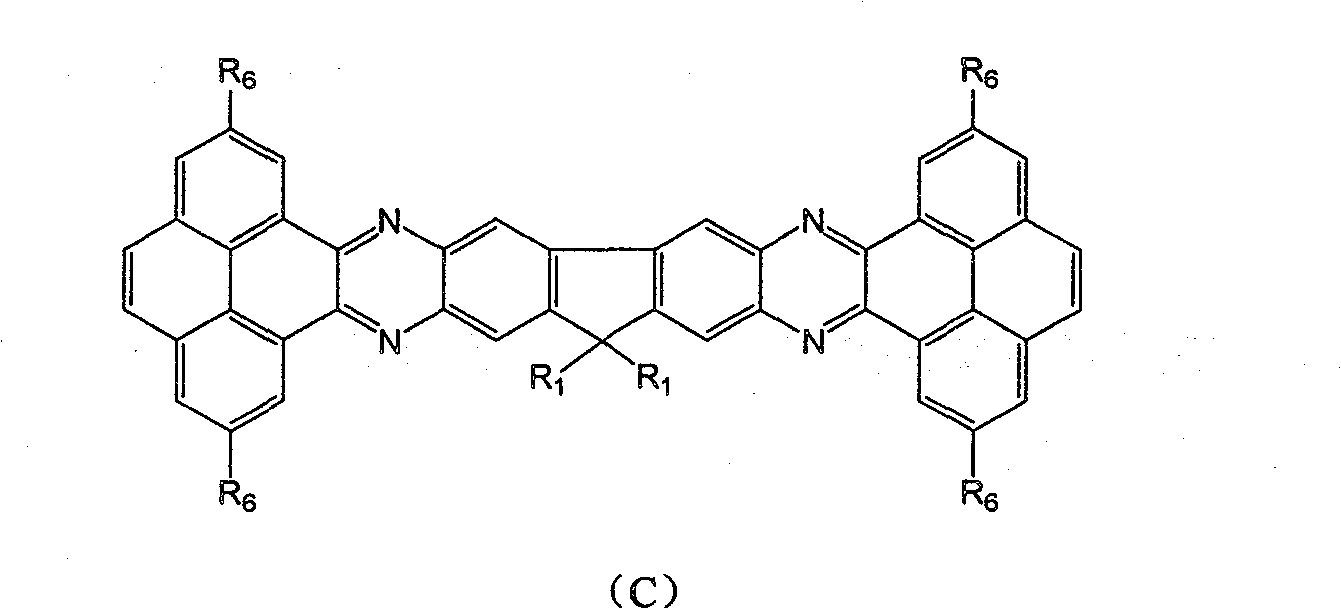
Fluorene derivative containing large conjugated molecule and preparation thereof
A technology for conjugated molecules and derivatives is applied in the field of fluorene derivatives and their preparation, and achieves the effects of increasing nonlinear optical coefficients, improving solubility and processing performance, and overcoming poor chemical stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] Add 1g of 2,3,6,7-tetraamino-9,9'-diisooctylfluorene, 3 times the molar amount of phenanthrenequinone and 1 times the molar amount of potassium carbonate to 15mL acetic acid solution, under the protection of nitrogen , Refluxed for 2h, cooled and filtered under reduced pressure to obtain 0.6g of yellow solid. MS: 794.4368; 1 H NMR (400M, CD 3 OD): δ( * 10 -6 )6.79(s, 2H), 6.50(s, 2H), 7.19-7.30(s, 8H), 1.89-1.92(m, 4H, CH 2 ), 1.02-1.08 (m, 4H, CH 2 ), 0.67(t, J(H-H)=7.4MHz, 6H), 0.52-0.63(m, 4H, CH 2 ).
Embodiment 2
[0043]
[0044]Add 1g of 2,3,6,7-tetraamino-9,9'-diisooctylfluorene, 2.5 times the molar amount of 3,6-dinitrophenanthrenequinone and 1 times the molar amount of sodium hydride to 20mL acetic acid solution , under the protection of nitrogen, reflux for 10 h, cooled and filtered under reduced pressure to obtain 0.8 g of yellow solid. MS: 974.3766; 1 H NMR (400M, CD 3 OD): δ( * 10 -6 )6.789(s, 2H), 6.53(s, 2H), 5.17(s, 8H), 1.90-1.93(m, 4H, CH 2 ), 1.28-1.33 (m, 20H, CH2), 1.20-1.25 (m, 46H, CH 2 ), 0.88(t, J(H-H)=7.3MHz, 6H).
Embodiment 3
[0046]
[0047] Add 1g of 2,3,6,7-tetraamino-9,9'-diisooctylfluorene, 2.2 times the molar amount of 2,7-dicyanophenanthrenequinone and 1.5 times the molar amount of calcium hydride to 15mL propionic acid The solution was refluxed for 20 h under the protection of nitrogen, cooled and filtered under reduced pressure to obtain 0.55 g of a yellow solid. MS: 894.4168; 1 H NMR (400M, CD 3 OD): δ( * 10 -6 )6.79(s, 2H), 6.50(s, 2H), 5.19(s, 8H), 1.84-1.89(m, 4H, CH 2 ), 1.62-1.70 (d, J=(H-H)=7.4MHz, 1H), 0.91 (d, J(H-H)=7.2MHz, 12H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com