New compound, preparation method and application thereof
A compound and plant technology, applied in the field of tumor pain and improving immunity, in the field of tumor treatment, can solve the problems of few researches on the chemical components of Baitu Ling, achieve the improvement of immunity, increase the count of white blood cells, and the control method is simple Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
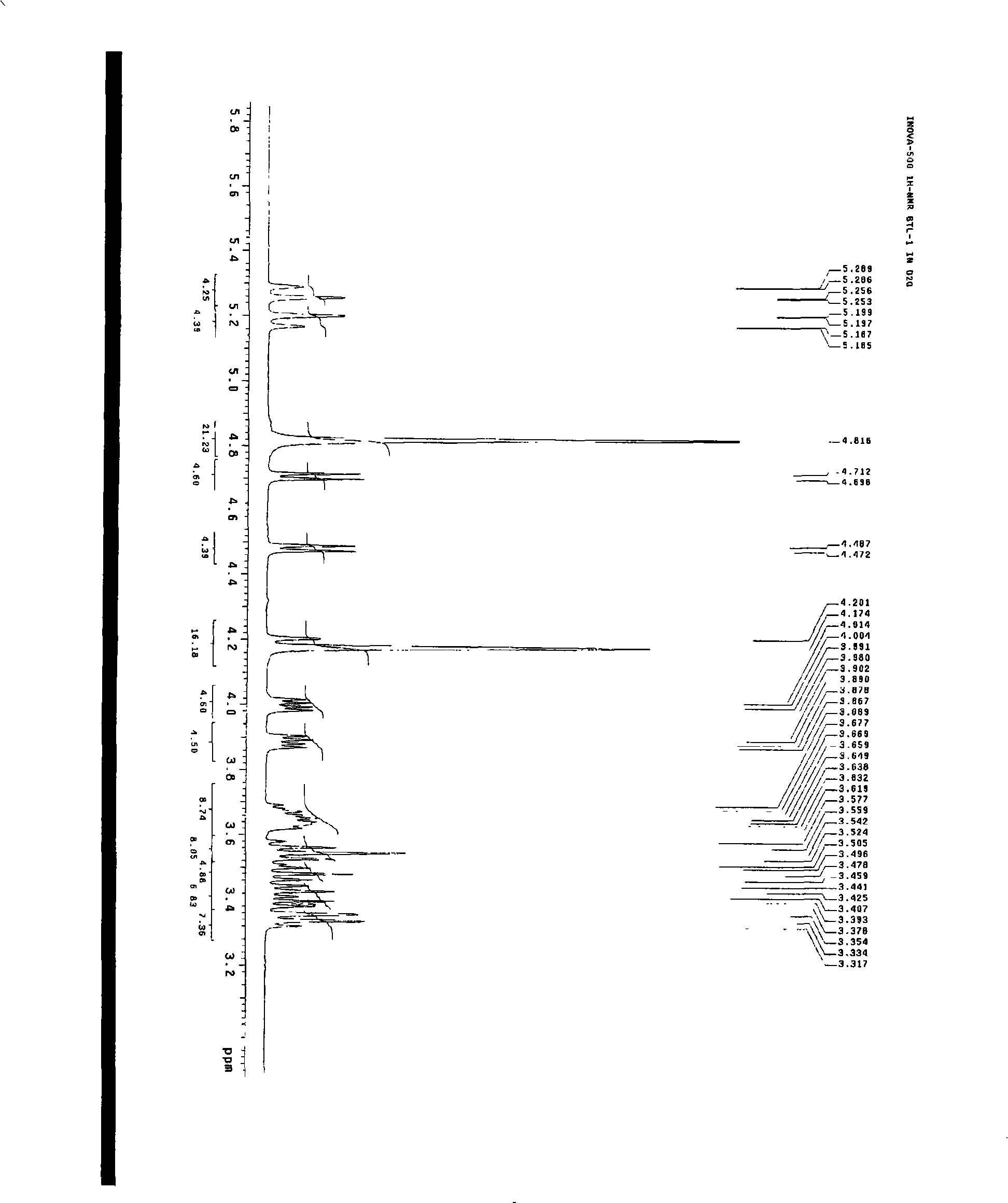
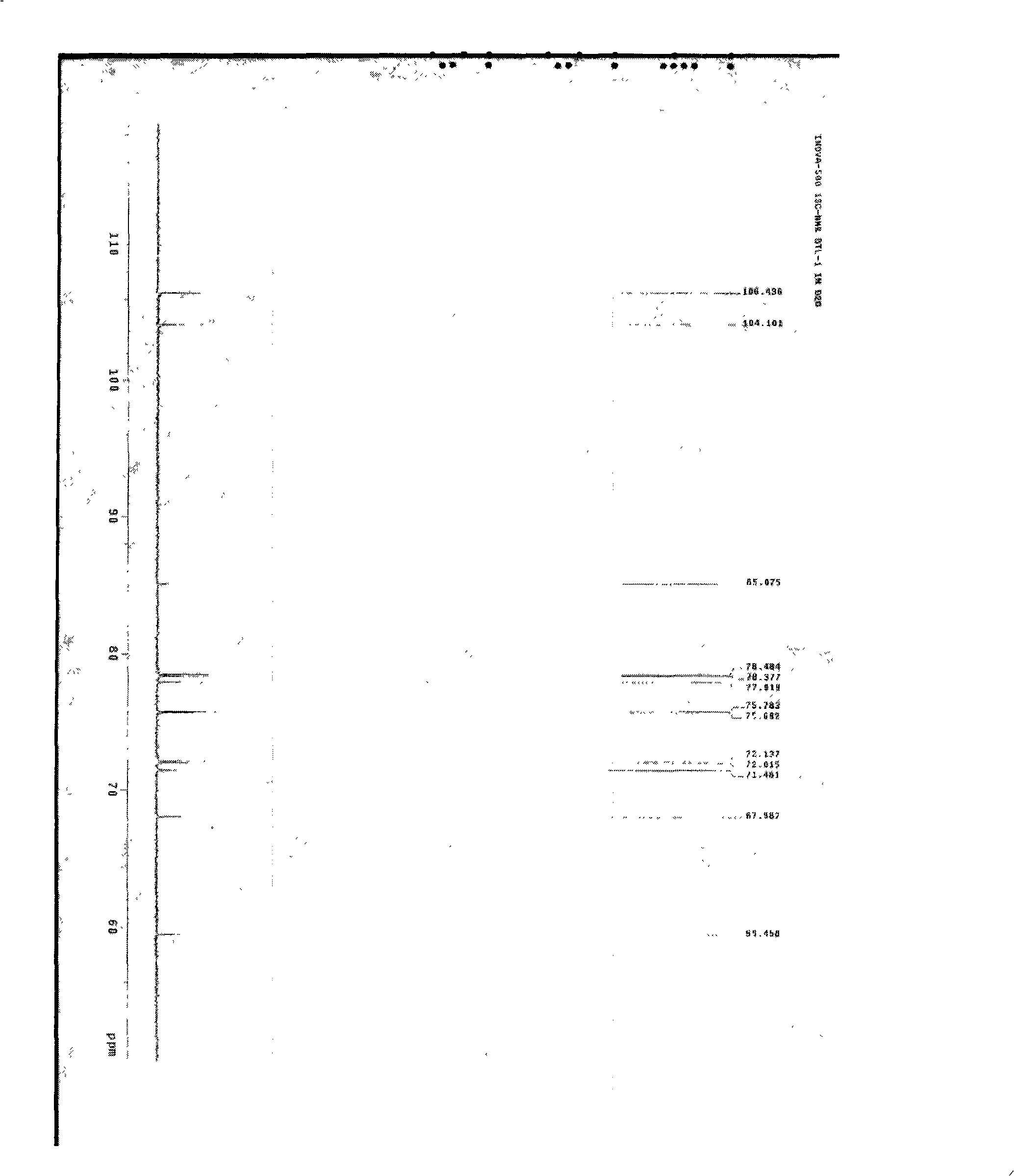
[0028] Preparation of compounds of the present invention
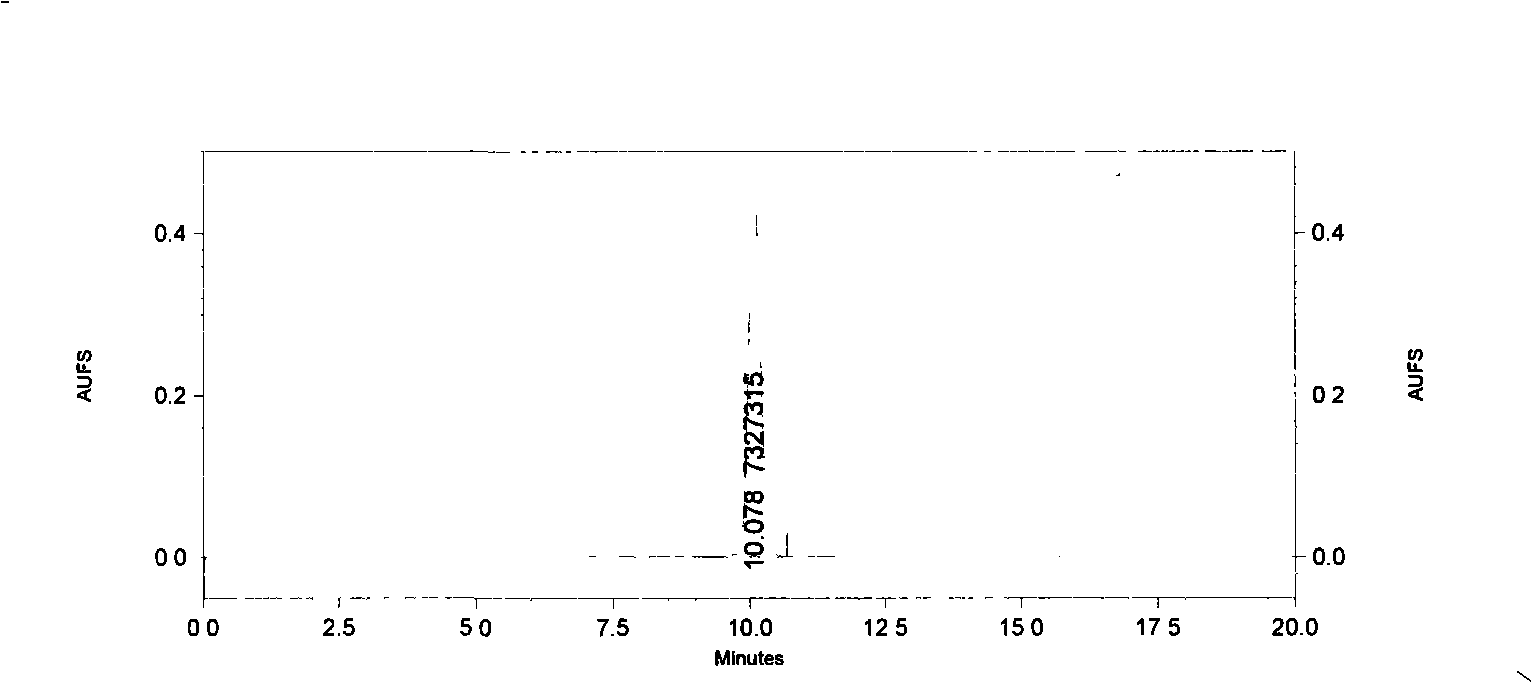
[0029] Dried Baituling medicinal material 2Kg, crushed to coarse powder. Add water and ultrasonically (33KHz) extract twice, each time 30min, add water 6 times, 4 times. The extract was filtered and centrifuged (4000r / min) to obtain a clear liquid. For super-porous resin HPD-100, the ratio of column height to discard is 1:10, the flow rate is 1BV / h (referring to 1 resin column volume per hour), and the water is eluted at 2BV / h. The eluate was detected by HPLC, and the target fractions were combined and evaporated to dryness under reduced pressure (60°C, -0.09Mpa) to obtain a crude product. The crude product was washed with methanol until the methanol liquid was colorless, filtered and dried under reduced pressure. The dried product under reduced pressure was dissolved with acetone:water (1:1), and filtered to obtain a clear liquid. Gradually increase the ratio of acetone to 2:1-5:1, and filter to obtain a clear liq...
Embodiment 2
[0063] The acute toxicity test of embodiment 2 compounds of the present invention
[0064] According to the pre-test results, 60 mice were randomly divided into 6 groups, 10 in each group. Wherein 1 group of mice is used for intraperitoneal injection route, one-time intraperitoneal injection of 2000mg / kg compound of the present invention; another 5 groups of mice are used for intravenous injection route, and the drug concentrations of each group are respectively 400, 344.8, 297.3, 256.3, 221mg / kg, one-time intravenous injection. Continuous observation was carried out for two weeks, and the toxicity and death of the animals were observed day by day. Calculation of LD by Bliss method 50 .
[0065] Administration route of intraperitoneal injection: 2000 mg / kg of the compound of the present invention was administered by intraperitoneal injection, and none of the 10 animals died. As a result, the LD of the compound of the present invention administered by intraperitoneal inject...
Embodiment 3
[0071] The antitumor effect of embodiment 3 compounds of the present invention
[0072] 50 mice, weighing 18-22 g, half male and half male. Sterile subcutaneous inoculation of S in the right axilla of each mouse 180 Cell suspension (5×10 6 Individuals / ml) 0.2ml, were randomly divided into 5 groups according to body weight the next day after inoculation, 10 / group. In the experiment, a model control group, a positive control group (CY 30 mg / kg), and 3 administration groups (20, 40, 80 mg / kg of the compound of the present invention) were set up. Animals in each group were administered 24 hours after inoculation. The model control group was intraperitoneally injected with normal saline 0.2ml / 10g body weight every day for 12 consecutive days; the positive control group was intraperitoneally injected with CY once every other day, a total of 6 times; Administration by intraperitoneal injection for 12 consecutive days. The mice in each group were weighed 24 hours after the last dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com