Composition containing fucoidan or fucoidan hydrolysate and immunopotentiating material
A fucoidan and hydrolyzate technology, which is applied in the directions of pharmaceutical combinations, plant raw materials, medical preparations containing active ingredients, etc., to achieve the effects of simple manufacture, synergistic enhancement of immune function regulation or immune activation, and easy handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0171] Preparation of fucoidan fraction
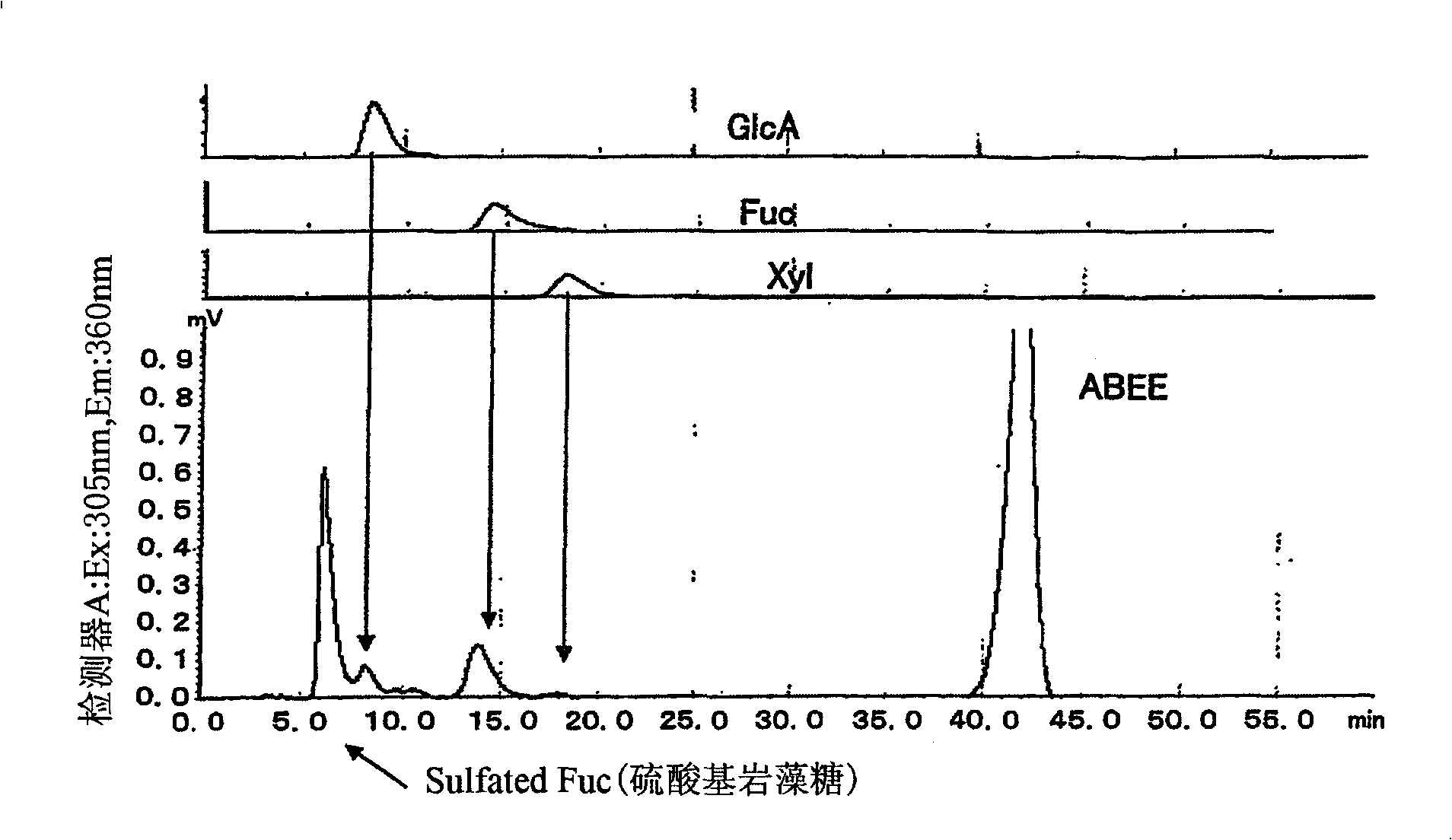
[0172] Add 1000ml of distilled water to 100g of mozuku algae in Okinawa, Japan, and extract at 100°C for 1 hour. After the obtained extract was cooled, suction filtration, electrodialysis (desalting) and freeze-drying were performed to obtain 2 g of fucoidan fraction. Make the fucoidan contain 2N H 2 SO 4 The aqueous solution at 100°C was hydrolyzed for 1 hour, the obtained aqueous solution was neutralized with 2N NaOH, and a sample for analysis of monosaccharides was prepared by labeling with ABEE fluorescence. It was confirmed that the composition of the sugar was as follows: SFuc (fucose sulfate): GlcA (glucuronic acid): Fuc (fucose): Xyl (xylose) = 49.3: 4.9: 12.1: 1 ( figure 1 ).
[0173] Chromatographic column: Cosmosil C18 AR-II (4.6mmφ×250mm)
[0174] Mobile phase: 0.2M potassium borate buffer containing 10% acetonitrile
[0175] Flow rate: 1.0ml / min
[0176] Temperature: 45°C
[0177] Detection: fluorescence detector (...
Embodiment 2
[0179] Production of fucoidan hydrolyzate and determination of immune function regulation
[0180] The hydrolyzate of fucoidan was obtained under various conditions described below, and the immune function regulation effect was investigated using the composition of the hydrolyzate and lactic acid bacteria.
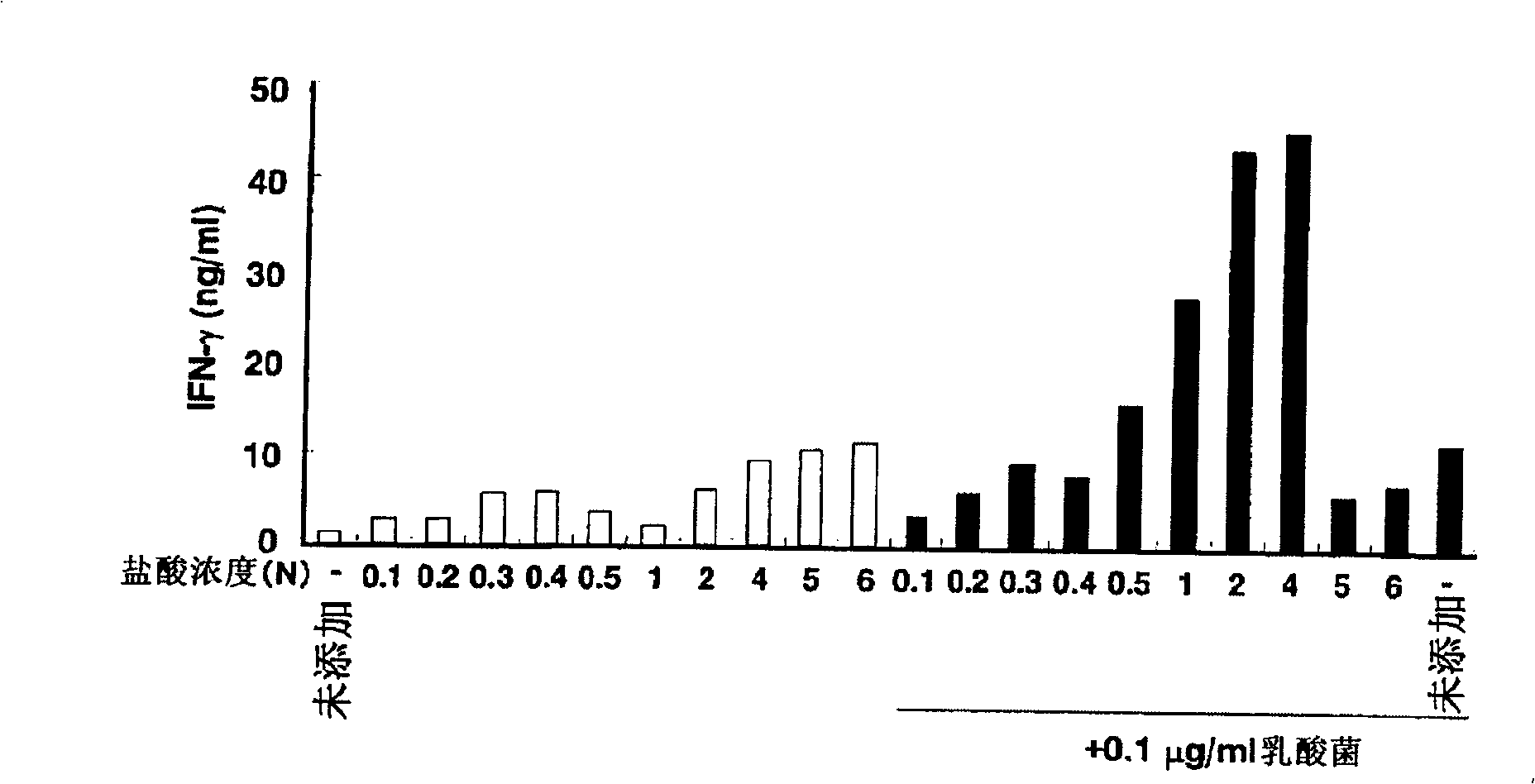
[0181] The Effect of Hydrolyzed Acid Concentration on the Modulatory Activity of Immune Function
[0182] The fucoidan obtained in Example 1 was hydrolyzed in 0.1, 0.2, 0.3, 0.4, 0.5, 1.0, 2.0, 4.0, 5.0, 6.0N HCl (80° C.) for 1 hour, and then 0.1, 0.2, After neutralization with 0.3, 0.4, 0.5, 1.0, 2.0, 4.0, 5.0, 6.0N NaOH, desalting by electrodialysis and freeze-drying, the hydrolyzate of fucoidan is obtained. The hydrolyzate was added at a concentration of 100 μg / ml to splenocytes (5 × 10 6 cells / ml). After 30 minutes, add 0.1 μg / ml lactic acid bacteria ( Lactobacillus pentosus ; FERM ABP-10028), cultivated for 24 hours, recovered the culture supernatant, and the p...
Embodiment 3
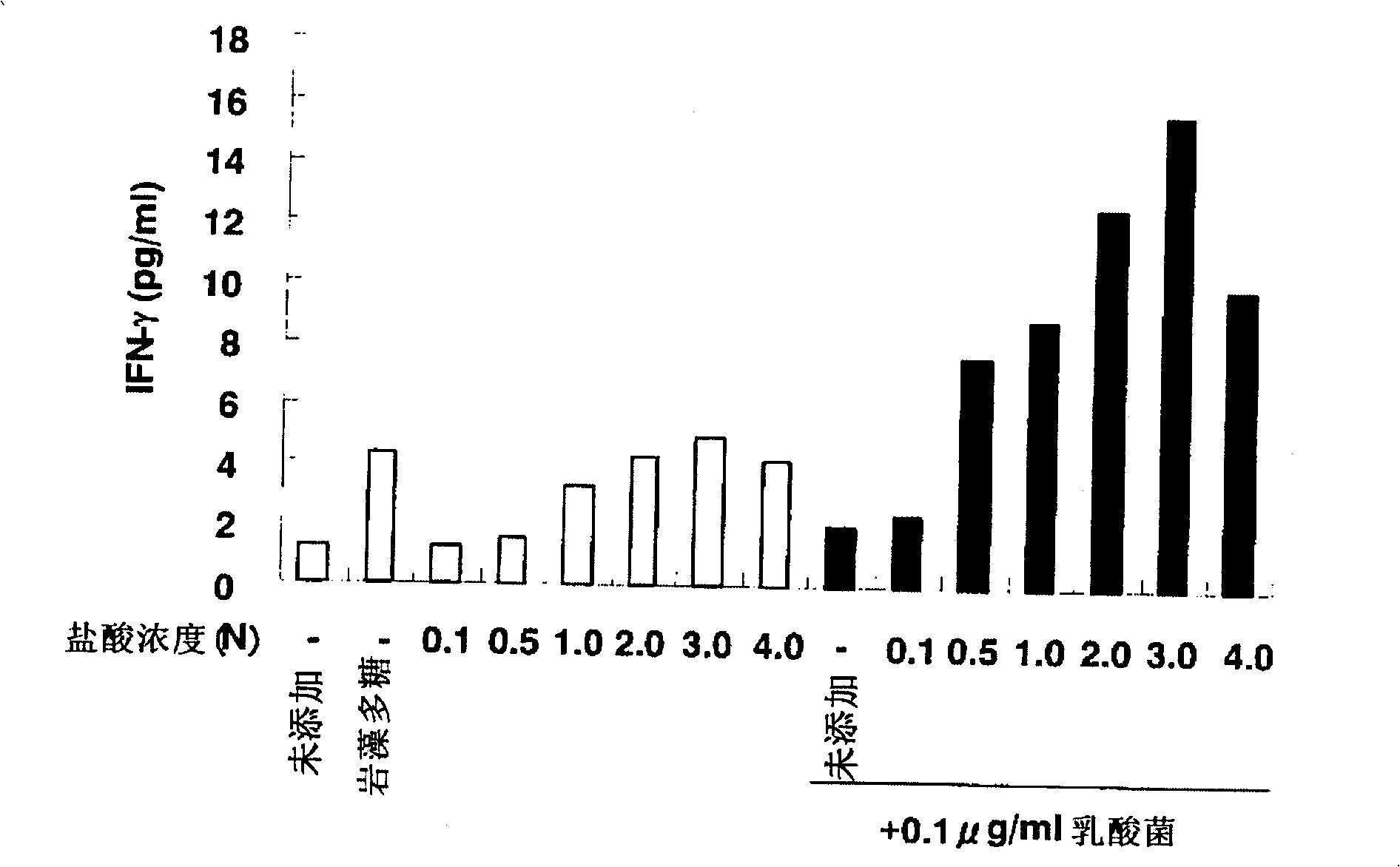
[0191] Regulatory Effects of Refined Fucoidan Hydrolyzate on Immune Function
[0192] Add 100ml of 2N HCl to 1g of fucoidan, and perform acid hydrolysis at 80°C for 1 hour. It was then neutralized with 1N NaOH. The resulting reaction solution was desalted by gel filtration (biogel P-6 (Bio-Rad)), and freeze-dried to obtain a component (oligosaccharide component) containing a fucoidan-oligosaccharide mixture. The resulting fucoidan-oligosaccharide mixture was subjected to chromatography using an anion exchange resin (Tosoh Corporation) activated with formic acid. As a result, the oligosaccharide fraction was separated into a fraction (Fr.B) containing oligosaccharides containing no sulfate groups obtained by elution with water, and a fraction containing oligosaccharides containing a large number of sulfate groups obtained by elution with 2N HCl. Components of sugars (sulfated sugar components). The sulfated sugar fraction was passed through gel filtration (Biogel P-6) and se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com