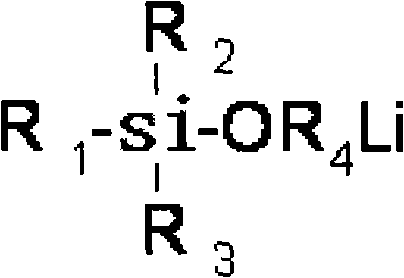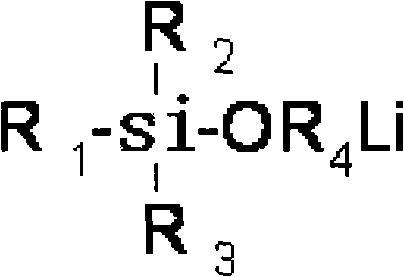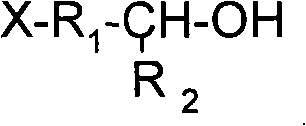Acyclic lithium alkylide evocating agent with hydroxylic protected by silane, preparation thereof and application thereof
A silane-protected, aliphatic technology, used in the synthesis of aliphatic alkyllithium initiators, high-performance hydroxyl-terminated polybutadiene, and heterofunctionalized polybutadiene, can solve the problem of reducing the average functionality of polymers , incomplete hydrolysis of protecting groups, many preparation steps, etc., to achieve high yield, good storage stability, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In a 250ml three-necked flask, 38g (0.4mol) of 3-chloro-1-propanol, 61g (0.4mol) of triethylchlorosilane, and 80ml of N,N-dimethylformamide were sequentially added. Then the N, N-dimethylformamide (30ml) solution containing 38g (0.44mol) imidazole was added dropwise to the reaction system within 30min at 5°C. 2 Stir at room temperature under protection for 6 hours. 350ml of cyclohexane was added, and then washed three times with 5% aqueous sodium bicarbonate solution, and the cyclohexane layer was separated. After drying with calcium hydride, the solvent was evaporated by rotary evaporation, and the colorless liquid was obtained by chromatographic column separation—namely, triethyl-(3-chloropropoxy)-silane, with a yield of 90%.
[0036] Under Ar atmosphere, add 8g (1.01mol) lithium, 100ml cyclohexane and 22.3g (0.107mol) triethyl-(3- Chloropropoxy)-silane was added into the reaction flask, the temperature was raised to 60°C, and stirred for 2h. The crude product was ...
Embodiment 2
[0040] In a 250ml three-necked flask, add 38g (0.4mol) 1-chloro-2-propanol, 77g (0.4mol) triisopropylchlorosilane, 100ml N, N-dimethylformamide and 35.2g (0.44 mol) pyrimidine, reacted at 5°C for 30 minutes, under N 2 Stir at room temperature under protection for 6 hours. Add 350ml of n-hexane, then wash with 5% NaHCO3 aqueous solution three times, and separate the n-hexane layer. After drying with calcium hydride, the solvent was evaporated by rotary evaporation, and the colorless liquid was obtained by separation through chromatographic column—that is, triisopropyl-(3-chloropropoxy)silane, with a yield of 83%.
[0041]Under Ar atmosphere, add 8g (1.01mol) lithium, 100ml cyclohexane and 22.3g (0.107mol) triisopropyl-(3 -Chloropropoxy)silane was added into the reaction flask, the temperature was raised to 60°C, and stirred for 2h. Crude product is filtered under Ar environment, obtains expected initiator, and its structural formula is as follows:
[0042] (CH...
Embodiment 3
[0045] In a 250ml three-necked flask, add 38g (0.4mol) 3-chloro-1-propanol, 60g (0.4mol) tert-butyldimethylsilyl chloride, 100ml N, N-dimethylformamide and 38g ( 0.44mol) of imidazole, reacted at 5°C for 30 minutes, and stirred at room temperature for 6 hours under nitrogen protection. Add 350ml of n-hexane, then wash with 5% aqueous sodium bicarbonate solution three times, and separate the n-hexane layer. After drying with calcium hydride, the solvent was evaporated by rotary evaporation, and the colorless liquid—tert-butyl-(3-chloropropoxy)dimethylsilane was obtained by chromatographic column separation, with a yield of 90%.
[0046] Under Ar atmosphere, add 8g (1.01mol) of lithium and 50ml of cyclohexane into the reaction flask into a 250ml three-necked flask equipped with a condenser, a constant pressure dropping funnel, and magnetic stirring, and then add 22.3g (0.107mol) of t 50 ml of a cyclohexane solution of butyl-(3-chloropropoxy)dimethylsilane was dropped into the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com