Immunity colloidal gold test paper strip for detecting and staphylococcal enterotoxin A and its production method
A colloidal gold test paper and colloidal gold technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that the detection process is limited in the laboratory, the detection takes a long time, and the operation process is complicated, so as to prevent the spread of the epidemic , maintaining human health and ensuring food safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: The connection relationship of the staphylococcus aureus enterotoxin A immune colloidal gold test strip is:
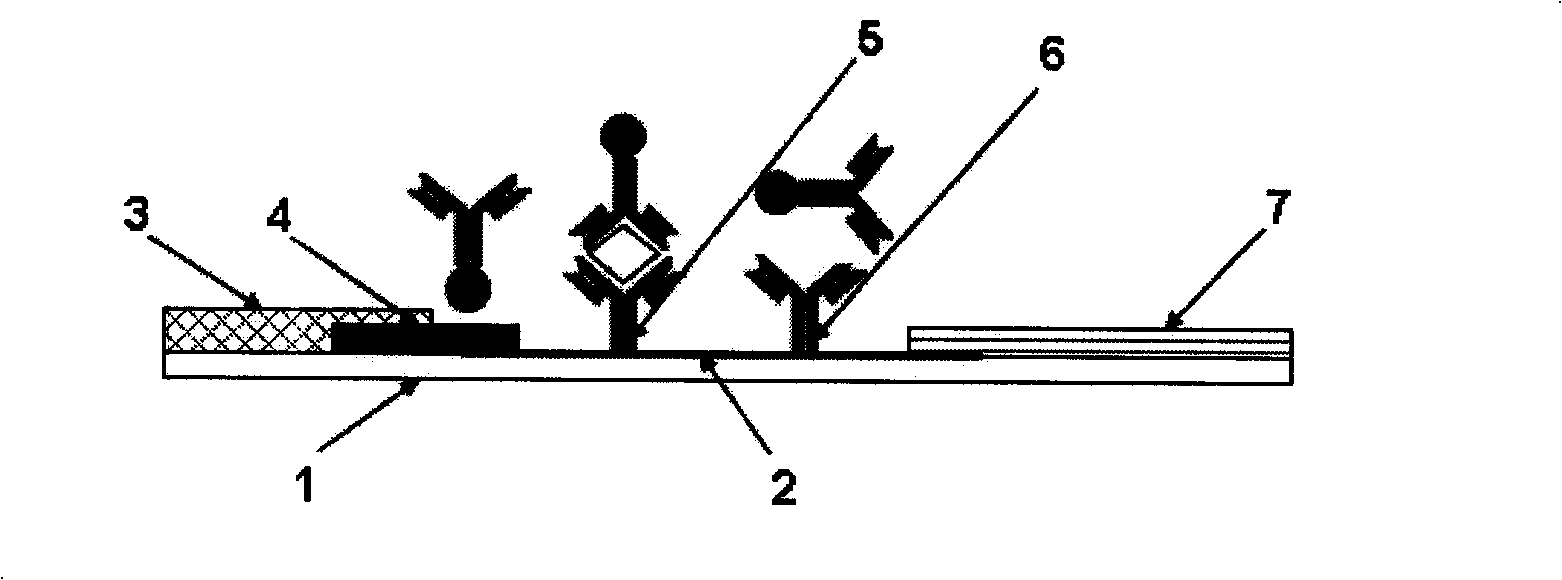
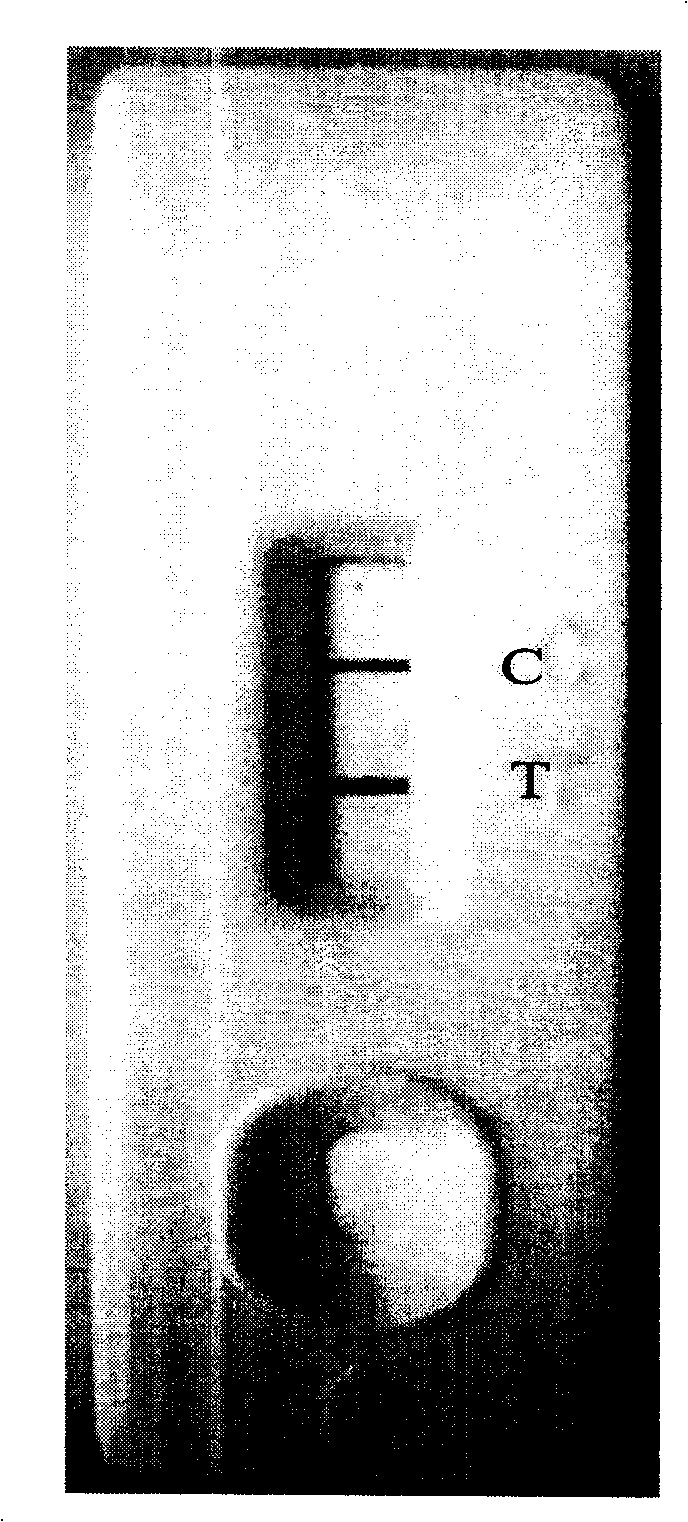
[0039] according to figure 1 It can be seen that it includes a sample pad 3, a gold standard pad 4 coated with a colloidal gold-labeled anti-SEA antibody, a nitrocellulose membrane coated with an anti-SEA antibody and goat anti-mouse IgG at a detection line 5 and a quality control line 6, Absorbent pad 7, a staphylococcus aureus enterotoxin A immune colloidal gold test strip whose connection relationship is: a PVC base plate 1 of appropriate size; at both ends of the PVC base plate 1, a sample pad 3 and an absorption pad 7 are respectively provided; The middle part of the PVC bottom plate 1 is provided with a nitrocellulose membrane detection layer 2, and at the junction of the nitrocellulose membrane detection layer 2 and the sample pad 3, a gold standard pad 4 coated with a gold-marked anti-SEA antibody is provided, and the gold standard pad 4 O...
Embodiment 2
[0040] Embodiment 2: produce the isolation of SEA staphylococcus aureus standard strain
[0041] In this example, Staphylococcus aureus (Zhang Wenli., etc. Different culture methods have influence on the results of Staphylococcus aureus enterotoxin. Chinese Journal of Health Inspection, 2004, 14(1): 108) was selected as the screening strain. According to the known gene sequence of Staphylococcus aureus enterotoxin A (SEA) (GenBank accession number: M28521), use Primer5.0 to design the upstream primer sea F: 5′GCC GCT AGC ATG AAA AAA ACA GCA TTT ACA TTA C 3′, (the underline is the Nhe I restriction site); downstream primer sea R: 5′CGC C GT CGA C TT AAC TTG TAT ATA AAT ATATAT CAA 3', (the underline is the Sa / I restriction site).
[0042] Inoculation of Staphylococcus aureus (Zhang Wenli., etc. Different culture methods have effects on the results of Staphylococcus aureus enterotoxin. Chinese Journal of Health Inspection, 2004, 14 (1): 108) in 7.5% sodium chloride broth, 37 ...
Embodiment 3
[0043] Embodiment 3: the preparation of SEA:
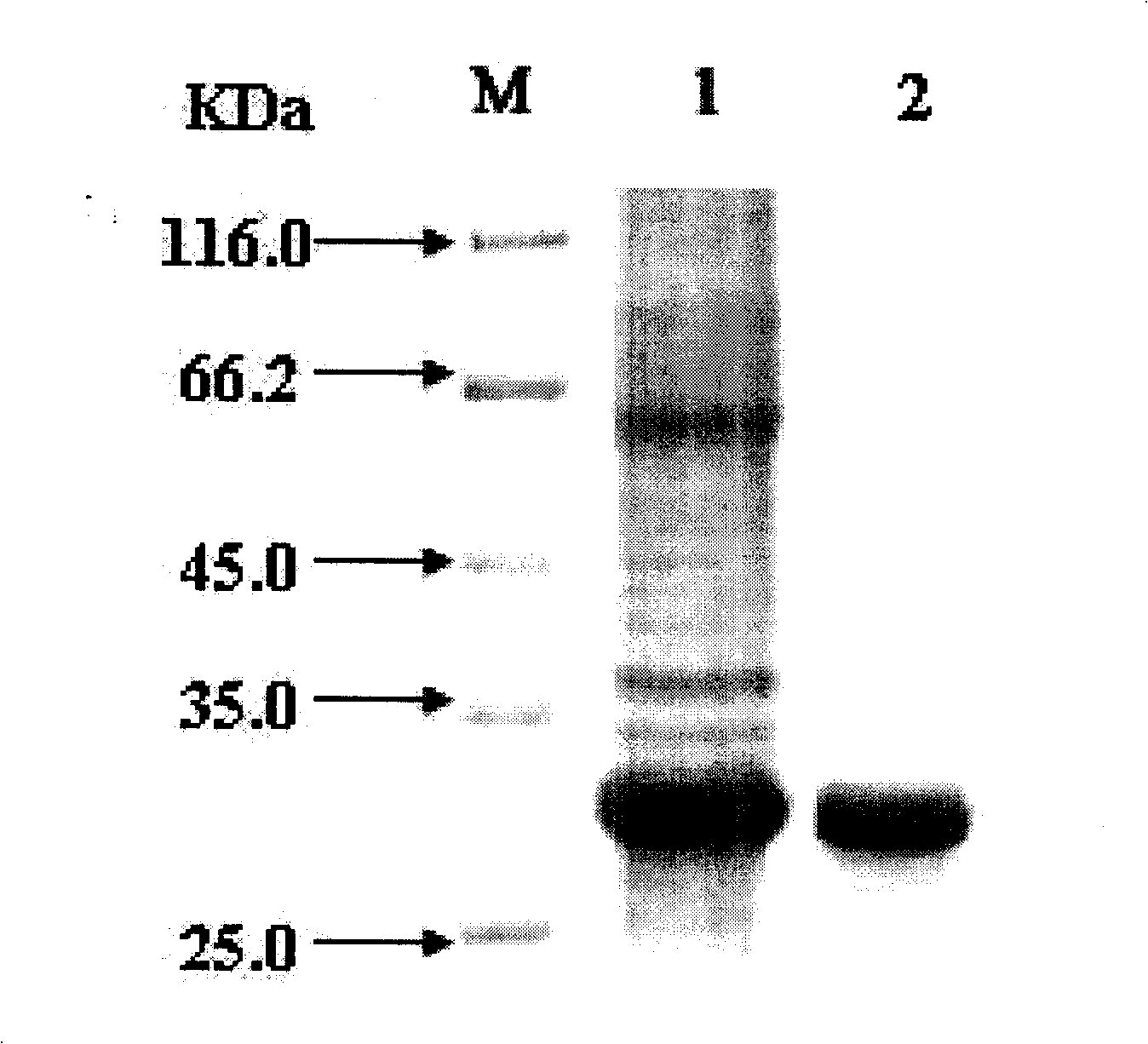
[0044] Extract the genomic DNA of the enterotoxigenic Staphylococcus aureus standard strain, and use it as a template to amplify the product by PCR. After the PCR product is purified by DNA Extraction Kit (Fermentas Company), it is carried out with the pGEM-T Easy cloning vector (Promega Company). Ligation, transformation, sequencing, the recombinant plasmid containing the correct sequence of SEA was digested by Nhel and SalI, and then ligated and transformed with the pET28a (Novagen Company) expression vector of the same double digestion, and the recombinant plasmid pET-SED was transformed into Escherichia coli BL21 ( DE3), induced by 1 mM IPTG at 37°C for 6 hours, and the cells were collected by centrifugation. Add 50mM pH8.0 Tris-HClbuffer according to the amount of bacterial weight 1 (g): 10 (ml), and ultrasonically destroy the bacteria on ice water. 4°C, 5000rpm, centrifuge for 10min, collect supernatant and precipitate resp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com