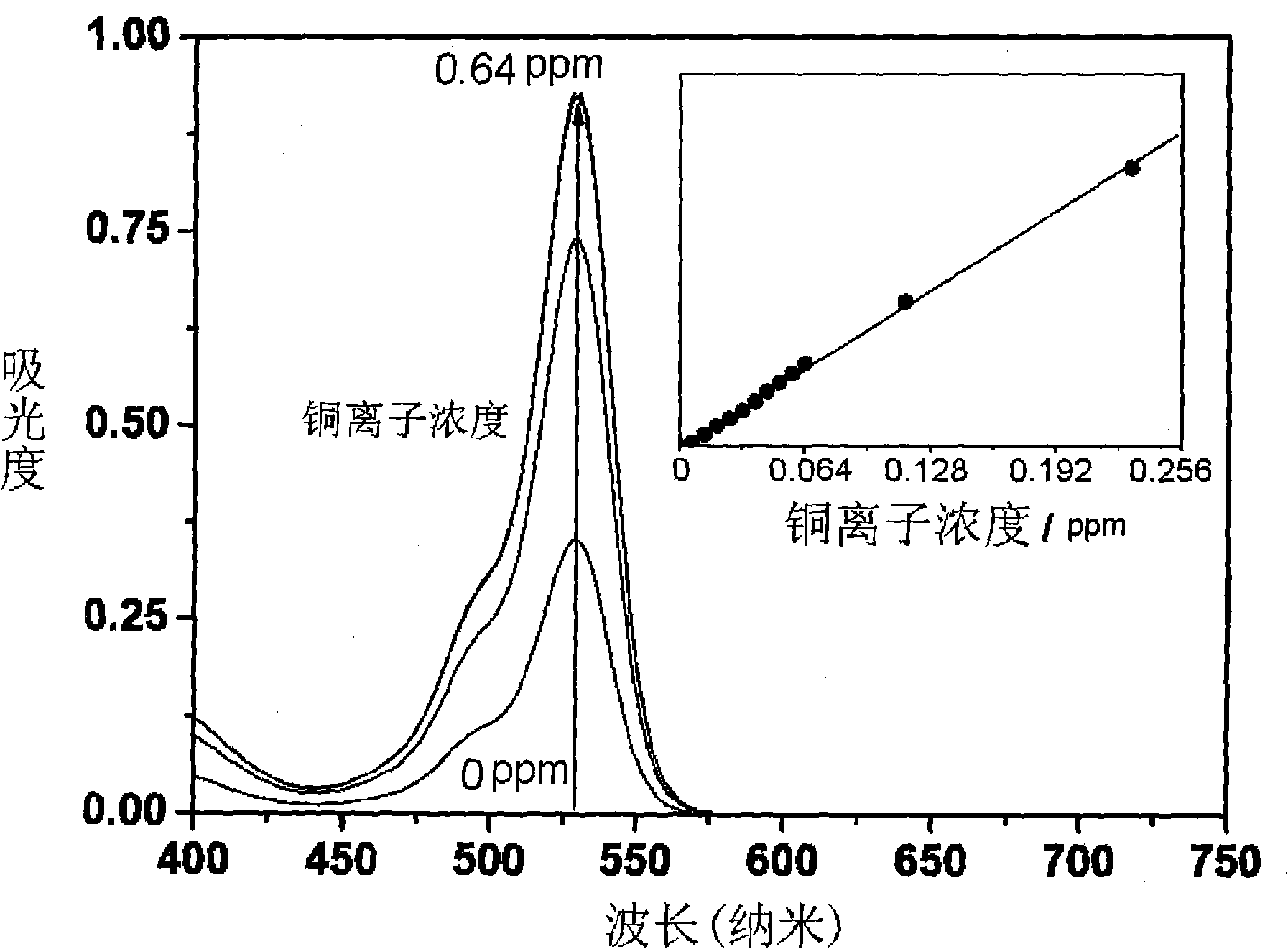
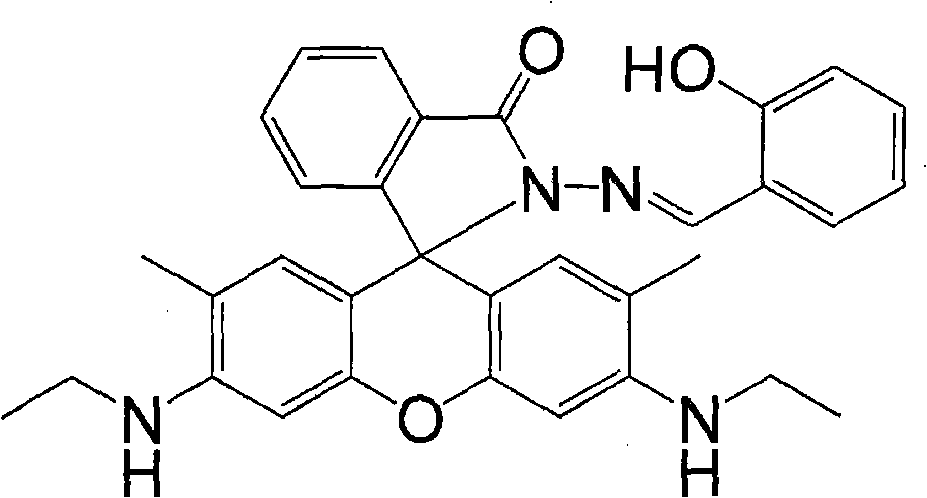
Rhodamine 6G hydrazide salicylaldehyde azomethine, synthesizing process and application in measuring content of copper ion
A synthesis method, hydrazide technology, applied in the field of organic dye solution production solution, can solve the problems of easy introduction of errors, large interference of transition metal ions, cumbersome operation, etc., and achieve low cost, wide linear range and simple manufacture.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Add 0.24 g of rhodamine 6G and 0.96 g of hydrazine hydrate in sequence to 15 ml of ethanol, react at 30 degrees Celsius for 8 hours, cool, and filter out the resulting precipitate. 0.043 g of the resulting precipitate was dissolved in 30 ml of ethanol / dichloromethane mixed solvent with a volume ratio of 3:1, 0.172 g of salicylaldehyde was added, reacted at 50 degrees Celsius for 12 hours, the solvent was removed under reduced pressure, and the solid residue was washed with ethanol. The product was dried to obtain rhodamine 6G hydrazide salicylaldehyde Schiffer base with a yield of 52%.
Embodiment 2
[0040] Add 24 grams of rhodamine 6G and 24 grams of hydrazine hydrate successively in 500 milliliters of ethanol, react at 80 degrees centigrade for 48 hours, cool, and filter out the precipitate produced. 21.5 grams of the resulting precipitate were dissolved in 300 milliliters of ethanol / dichloromethane mixed solvent with a volume ratio of 1:3, 21.5 grams of salicylaldehyde was added, reacted at 75 degrees Celsius for 96 hours, the solvent was removed under reduced pressure, and the solid residue was washed with ethanol. The product was dried to obtain rhodamine 6G hydrazide salicylaldehyde Schiffer base with a yield of 91%.
Embodiment 3
[0042] 2.4 g of rhodamine 6G and 0.6 g of hydrazine hydrate were added successively to 50 ml of ethanol, reacted at 60 degrees Celsius for 24 hours, cooled, and filtered out the resulting precipitate. 2.15 grams of the resulting precipitate were dissolved in 30 milliliters of ethanol / dichloromethane mixed solvent with a volume ratio of 1:1, 1.08 grams of salicylaldehyde was added, reacted at 75 degrees Celsius for 24 hours, the solvent was removed under reduced pressure, and the solid residue was washed with ethanol. The product was dried to obtain rhodamine 6G hydrazide salicylaldehyde Schiffer base with a yield of 78%.
[0043] Embodiment four to ten are the solutions of the present invention used for the determination of copper ion content in water samples.
[0044] The following solutions were prepared, numbered A, B, C, D, E, F, G, H, I in sequence.
[0045] A:
[0046] 495 grams of ethanol, 5 grams of acetic acid / sodium acetate buffer solution of pH=5 (the total concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com