Pharmaceutical composition containing beta-lactamase restrainer and piperacillin sodium with steady content and preparation method thereof
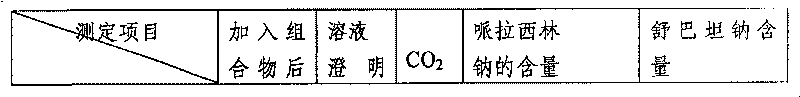
A technology of lactamase and composition, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, active ingredients of heterocyclic compounds, etc., can solve the problems of polluting liquid medicine, increasing drug pollution, milky white turbidity, etc., and achieve the elimination of medical treatment Hidden dangers of accidents, remarkable clinical curative effect, and the effect of solution clarification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add piperacillin sodium 100g, sulbactam sodium 100g and mannitol 50g in 100ml of water for injection, stir and make it dissolve, measure the pH value, add 115ml with the aqueous solution of sodium hydroxide of 0.1mol / l concentration with titration, Adjust the pH to 6.5. Stir to dissolve completely, sterile filter, potting, freeze-drying in a freeze dryer, pre-freezing, decompression, sublimation, drying, plugging, and capping to obtain the freeze-dried preparation 100 of the pharmaceutical composition of the present invention. bottle.
Embodiment 2
[0029] Add 100 g of piperacillin sodium, 100 g of tazobactam sodium and 50 g of mannitol into 60 ml of water for injection, stir to dissolve it, measure the pH value, add 190 ml of sodium carbonate aqueous solution with a concentration of 0.1mol / l in a titration mode, adjust The pH is 6.4. Stir to dissolve, sterile filter, potting, freeze-drying in a freeze dryer, pre-freezing, decompression, sublimation, drying, plugging, and capping to obtain 100 bottles of the freeze-dried preparation of the pharmaceutical composition of the present invention .
Embodiment 3
[0031] With piperacillin sodium 100g, sulbactam sodium 50g and sorbitol 30g, add in the water for injection 90ml, stir and make it dissolve, measure pH value, add 110ml with the aqueous solution of sodium bicarbonate of 0.2mol / l concentration with titration, Adjust the pH to 6.3. Stir to dissolve, sterile filter, potting, freeze-drying in a freeze dryer, pre-freezing, decompression, sublimation, drying, plugging, and capping to obtain 100 bottles of the freeze-dried preparation of the pharmaceutical composition of the present invention .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com