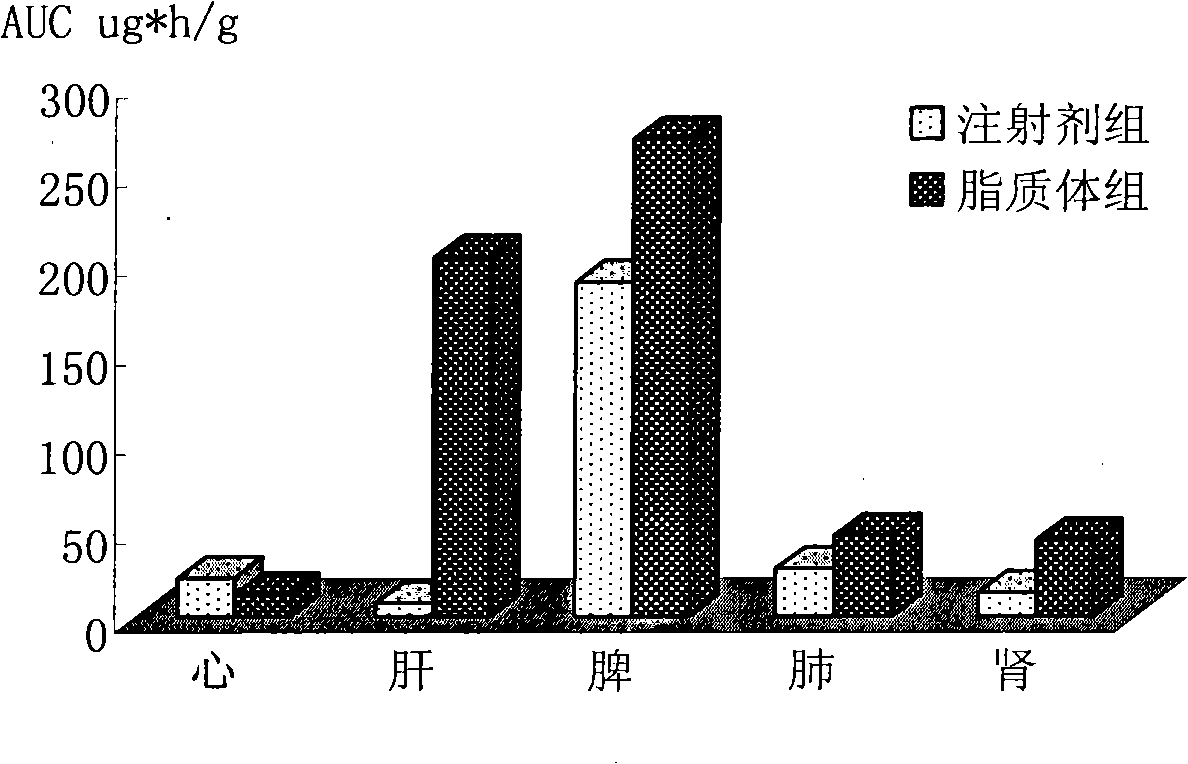
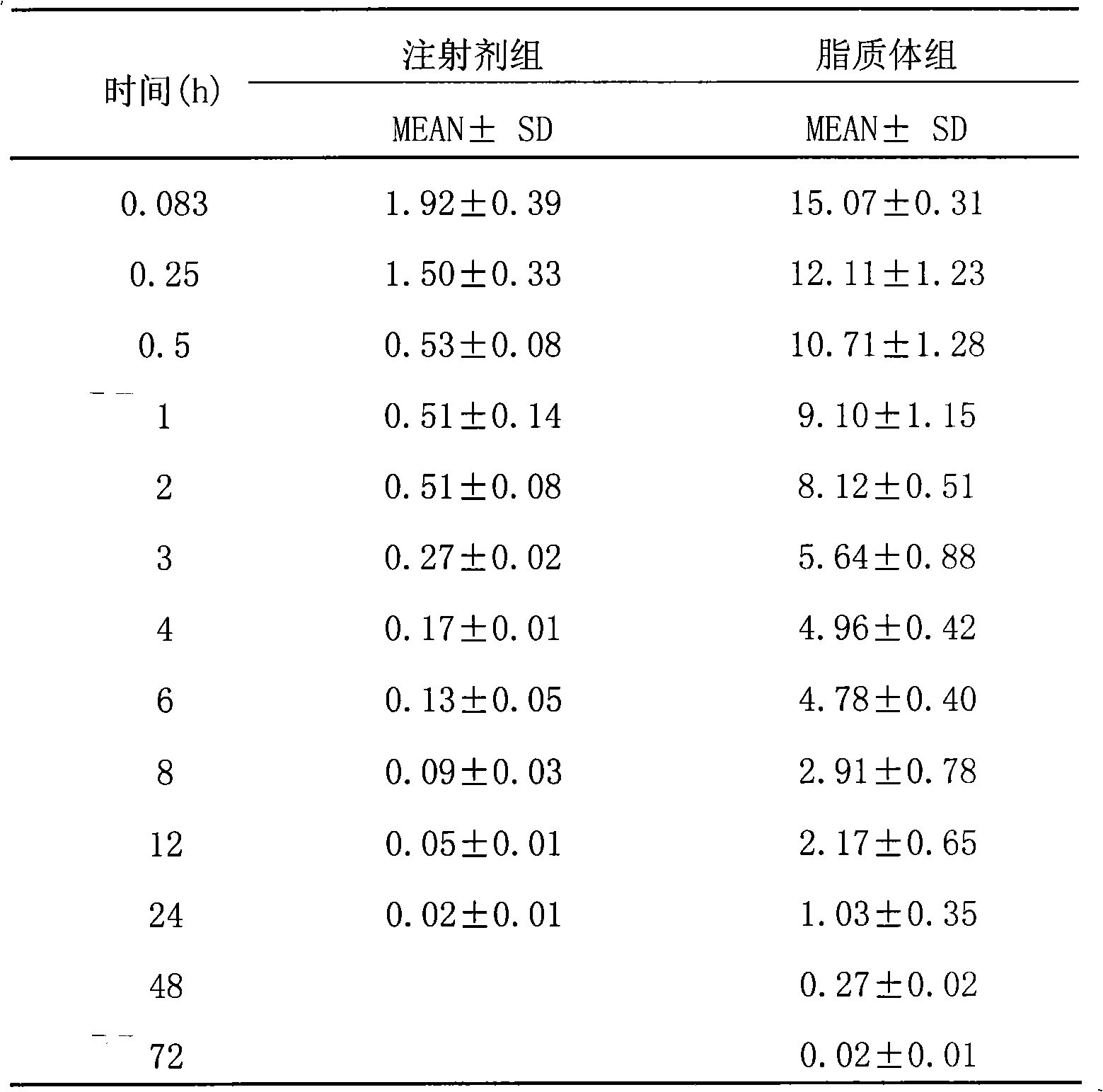
Epirubicin hydrochloride liposome and preparation thereof
A technology of epirubicin hydrochloride fat and epirubicin hydrochloride, which is applied in the field of epirubicin hydrochloride liposome and its preparation, can solve the problems of strong toxicity and side effects, reduce toxicity, reduce production costs, and be suitable for industrial The effect of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 prepares epirubicin liposome
[0049] Epirubicin 0.4mg
[0050]Hydrogenated Phospholipids 30mg
[0051] Cholesterol 11.5mg
[0052] Preparation method: Weigh the phospholipids and cholesterol in the above formula and dissolve them in an appropriate amount of chloroform / methanol solution to obtain a lipid solution. Evaporate under reduced pressure on a rotary evaporator to remove the organic solvent, and form a uniform lipid on the bottle wall. film, add 150mM ammonium sulfate solution to the lipid film, shake for 2h until the lipid film is completely hydrated, prepare blank liposomes with a micro squeezer, and elute the blank liposome solution with 10% sucrose solution through Replace the outer water phase with Sephadex G50 gel column, add the formulated amount of epirubicin hydrochloride sucrose solution, mix, put it in a water bath at 65°C and keep it warm for 20 minutes to obtain the product. It was determined that the encapsulation efficiency of the ...
Embodiment 2
[0053] Embodiment 2 prepares epirubicin liposome
[0054] Epirubicin 0.4mg
[0055] Soy Lecithin 30mg
[0056] DSPG 6mg
[0057] Cholesterol 11.5mg
[0058] Preparation method: Weigh the phospholipids and cholesterol in the above formula and dissolve them in an appropriate amount of chloroform / methanol solution to obtain a lipid solution. Evaporate under reduced pressure on a rotary evaporator to remove the organic solvent, and form a uniform lipid on the bottle wall. For thin film, add 150mM ammonium sulfate solution to the lipid film, shake for 2 hours until the lipid film is completely hydrated, use ultrasonic probe to reduce the particle size, and pass through the filter membranes with 0.22 μm and 0.15 μm pore sizes twice to obtain the liposome mixture. For the suspension, the blank liposome solution was eluted with 10% sucrose solution and passed through a Sephadex G50 gel column to replace the outer water phase, and the formula amount of epirubicin hydrochloride sucro...
Embodiment 3
[0059] Embodiment 3 prepares epirubicin liposome
[0060] Epirubicin 0.4mg
[0062] Dimyristate Phosphatidylglycerol 6mg
[0063] PEG 2000 -DSPE 7mg
[0064] Cholesterol 11.5mg
[0065] Preparation method: Weigh the phospholipids and cholesterol in the above formula and dissolve them in an appropriate amount of chloroform / methanol solution to obtain a lipid solution. Evaporate under reduced pressure on a rotary evaporator to remove the organic solvent, and form a uniform lipid on the bottle wall. film, add 150mM ammonium sulfate solution to the lipid film, shake for 2h until the lipid film is completely hydrated, prepare blank liposomes with a micro squeezer, and elute the blank liposome solution with 10% sucrose solution through Replace the outer water phase with the Sephadex G50 gel column, add the prescribed amount of epirubicin hydrochloride sucrose solution, mix, place in a water bath at 65°C for 20 minutes, and obtain the product. The en...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com