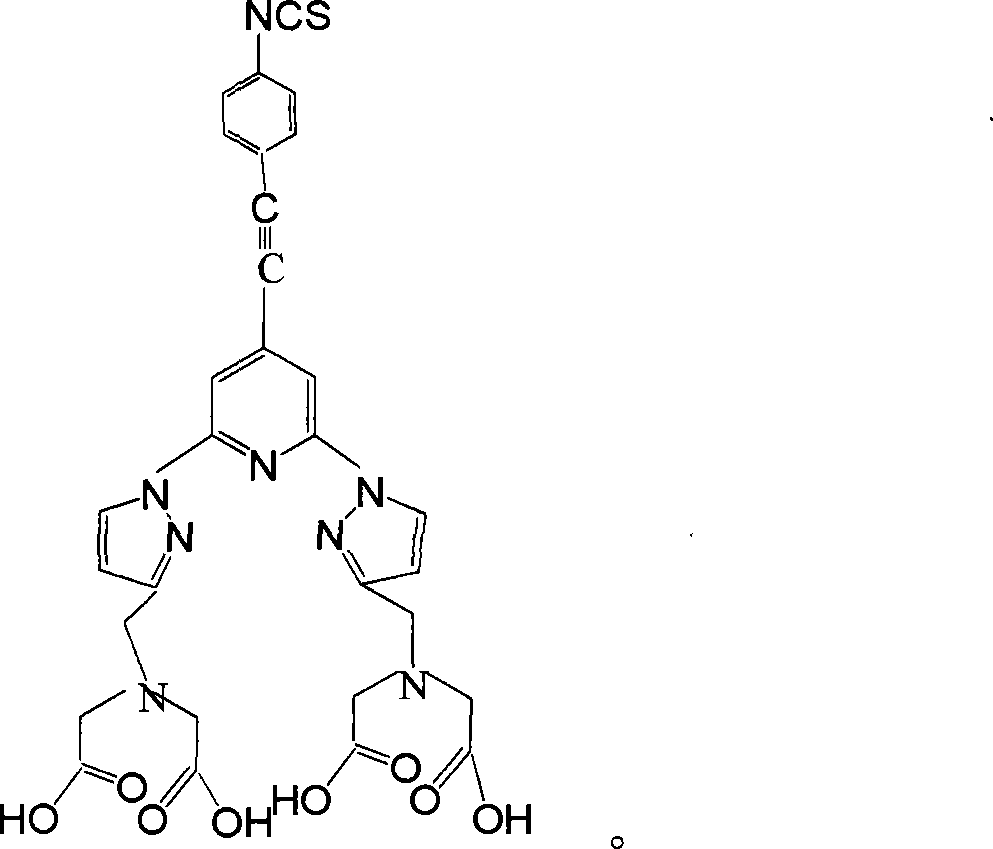
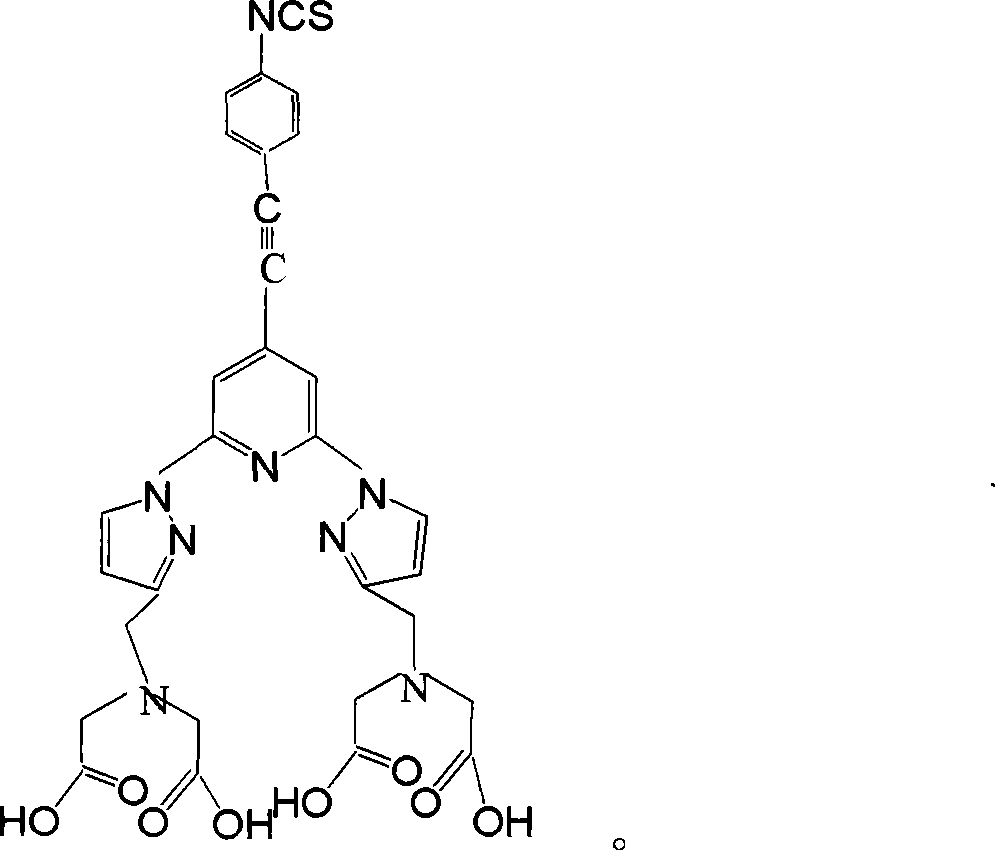
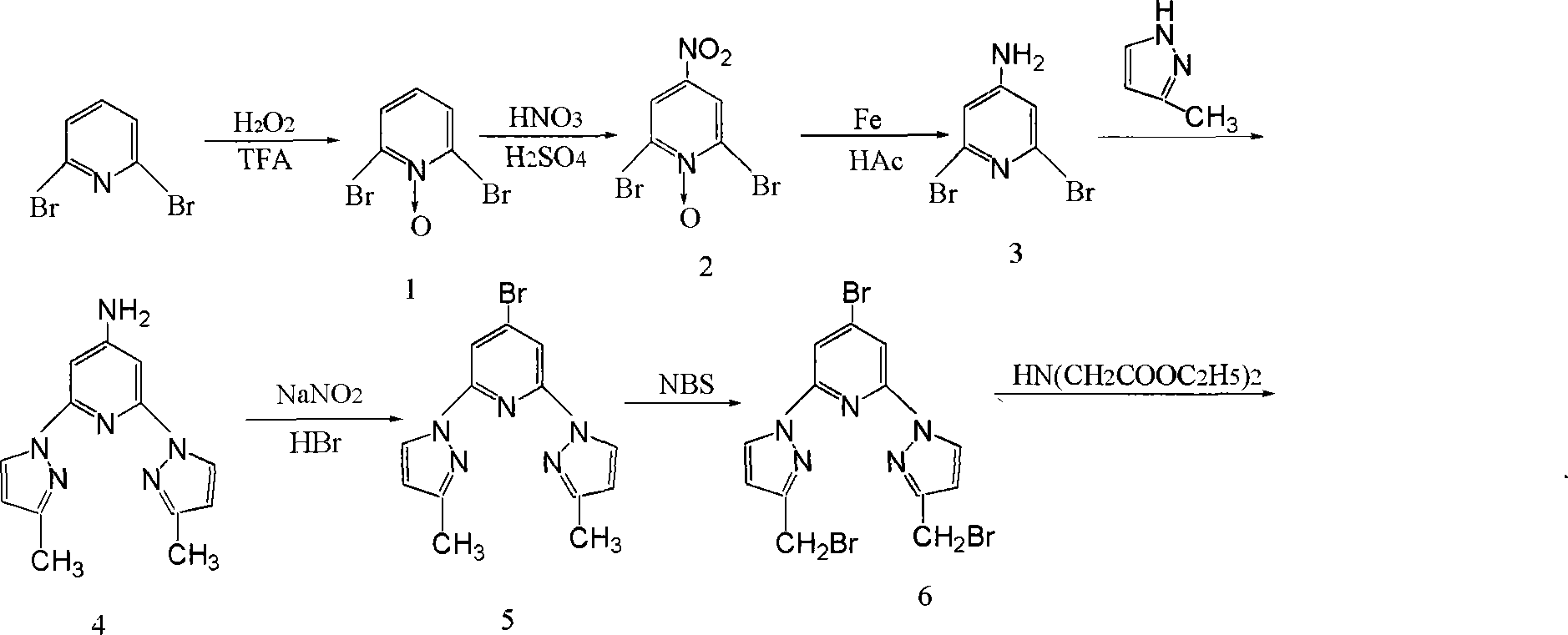
Homogeneous phase time discrimination fluorescence immunity analysis chelating agent and its preparing method
A time-resolved fluorescence and immunoassay technology, applied in chemical instruments and methods, luminescent materials, material testing products, etc., can solve the problems that the experimental application still has a certain distance, and the homogeneous immunoassay is limited to the laboratory stage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Synthesis of N-oxidized-2,6-dibromopyridine
[0043] Add 30.0g of 2,6-dibromopyridine, 180ml of trifluoroacetic acid, 40ml of hydrogen peroxide with a mass concentration of 30% to the reaction flask, stir and react at 35°C for 1h, cool to room temperature, pour into distilled water, and cool to -5 ℃, suction filtration, filtrate with anhydrous Na 2 CO 3 Neutralize to a pH value of 9, extract with chloroform, combine the organic phases, and evaporate the solvent to obtain a white solid product N-oxide-2,6-dibromopyridine.
[0044] (2) Synthesis of N-oxidation-2,6-dibromo-4-nitropyridine
[0045] Dissolve 19.8g of N-oxide-2,6-dibromopyridine in 67.8ml of concentrated H 2 SO 4 , add 67.8ml concentrated H under stirring 2 SO 4 And 34.2mL fuming nitric acid mixed acid, warming up to 80 ℃, stirring for 1h. After cooling, pour it into crushed ice, filter it with suction, and dry it in vacuo to obtain N-oxide-2,6-dibromo-4-nitropyridine as a light yellow solid produc...
Embodiment 2
[0063] (1) Synthesis of N-oxidation-2,6-dibromopyridine
[0064] Add 30.0g of 2,6-dibromopyridine, 190ml of trifluoroacetic acid, 40ml of hydrogen peroxide with a mass concentration of 30% to the reaction flask, stir and react at 38°C for 2h, cool to room temperature, pour into distilled water, and cool to -5°C , suction filtration, filtrate with anhydrous Na 2 CO 3 Neutralize to a pH value of 10, extract with chloroform, combine the organic phases, and evaporate the solvent to obtain a white solid product N-oxide-2,6-dibromopyridine.
[0065] (2) Synthesis of N-oxidation-2,6-dibromo-4-nitropyridine
[0066] Dissolve 19.8g of N-oxide-2,6-dibromopyridine in 67.8ml of concentrated H 2 SO 4 , add 67.8ml concentrated H under stirring 2 SO 4 And 34.2mL fuming nitric acid mixed acid, warming up to 90 ℃, stirring for 2h. After cooling, pour it into crushed ice, filter it with suction, and dry it in vacuo to obtain N-oxide-2,6-dibromo-4-nitropyridine as a light yellow solid pro...
Embodiment 3
[0084] (1) Synthesis of N-oxidized-2,6-dibromopyridine
[0085] Add 30.0g of 2,6-dibromopyridine, 200ml of trifluoroacetic acid, 40ml of hydrogen peroxide with a mass concentration of 30% to the reaction flask, stir and react at 42°C for 5h, cool to room temperature, pour into distilled water, and cool to -5 ℃, suction filtration, filtrate with anhydrous Na 2 CO 3 Neutralize to a pH value of 11, extract with chloroform, combine the organic phases, and evaporate the solvent to obtain a white solid product N-oxide-2,6-dibromopyridine.
[0086] (2) Synthesis of N-oxidation-2,6-dibromo-4-nitropyridine
[0087] Dissolve 19.8g of N-oxide-2,6-dibromopyridine in 67.8ml of concentrated H 2 SO 4 , add 67.8ml concentrated H under stirring 2 SO 4 And 34.2mL fuming nitric acid mixed acid, warming up to 100 ℃, stirring for 3h. After cooling, pour it into crushed ice, filter it with suction, and dry it in vacuo to obtain N-oxide-2,6-dibromo-4-nitropyridine as a light yellow solid prod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com