Dexibuprofen pharmaceutical composition with improved dissolving out capability and method for preparing the same
The technology of a composition and a pharmaceutical preparation is applied in the field of dexibuprofen pharmaceutical composition and its preparation, which can solve the problems of slow dissolution of dexibuprofen, reduce gastrointestinal ulcer bleeding and improve plasma bioavailability , the effect of reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
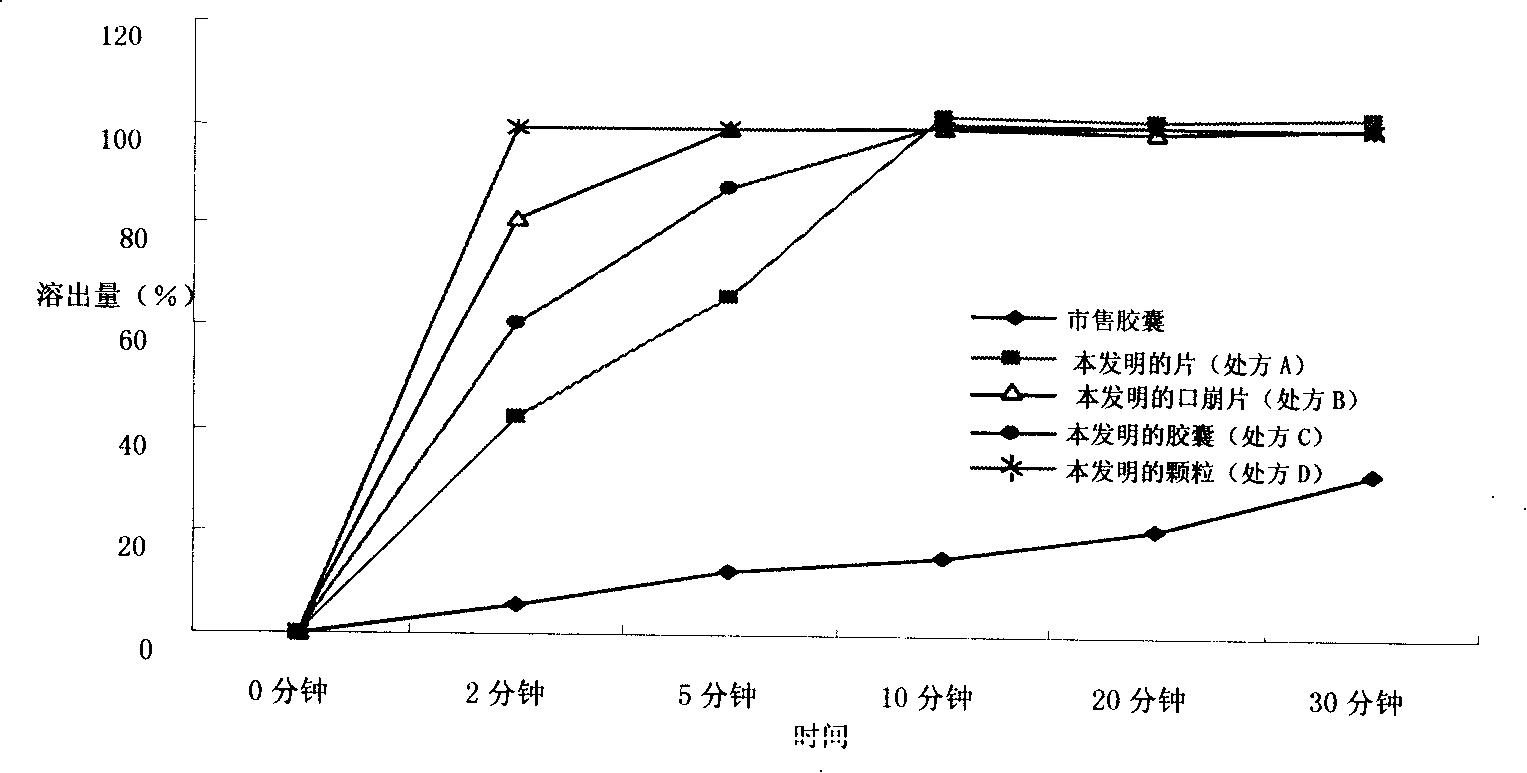
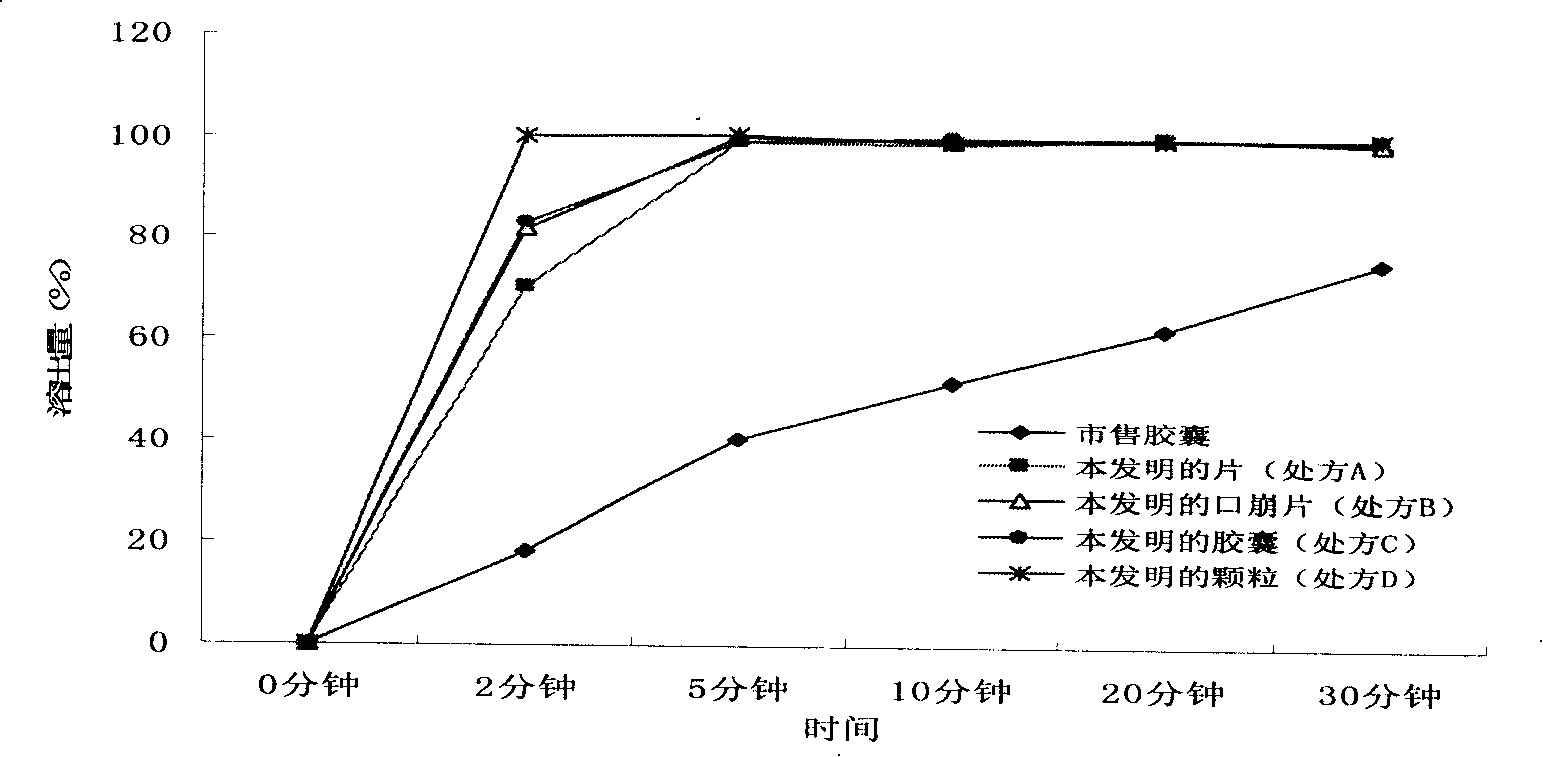
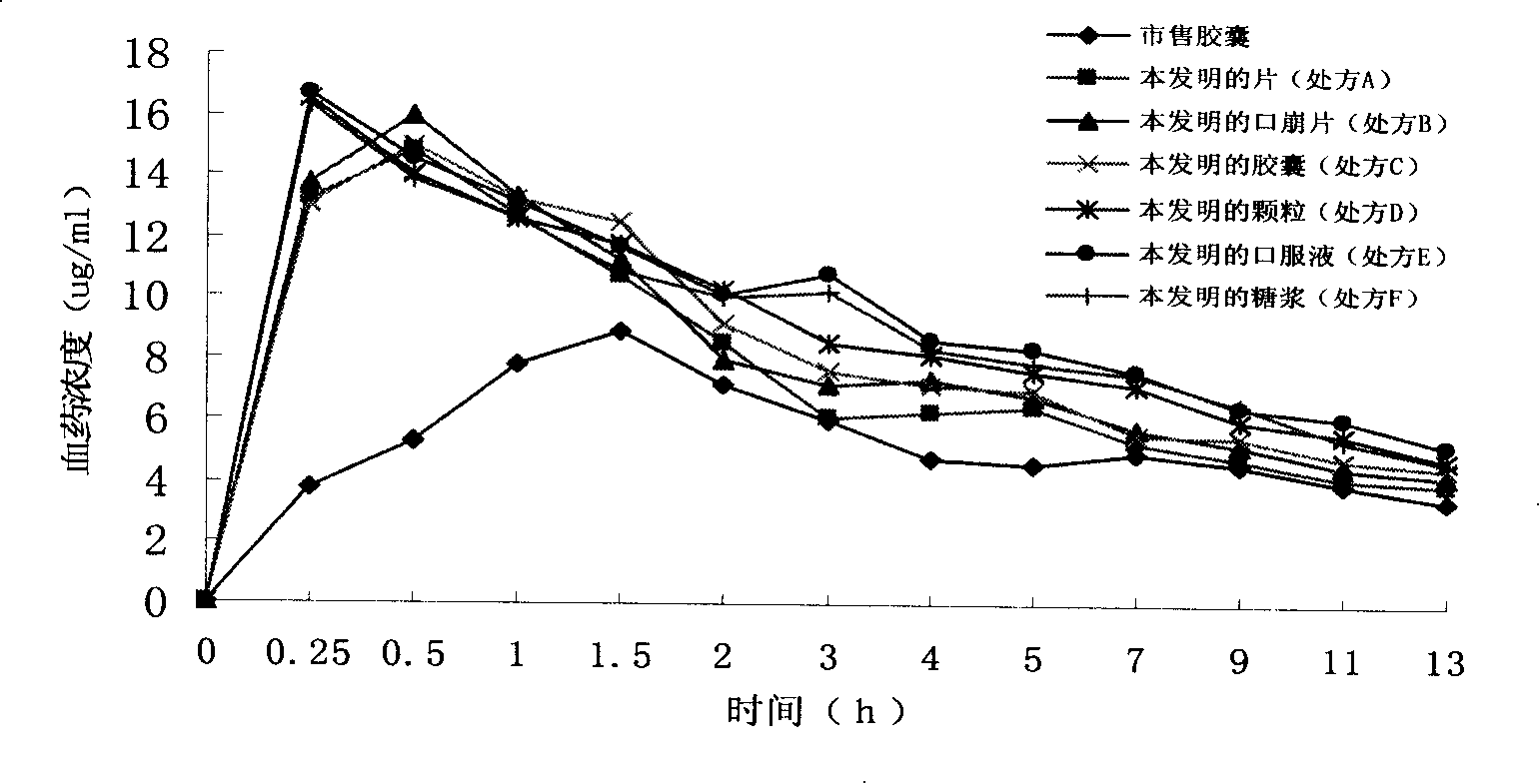
Image
Examples
Embodiment 1
[0056] Embodiment 1 Preparation of Dexibuprofen pharmaceutical composition improving dissolution performance of the present invention
[0057] 1. Prescription A (tablet)
[0058] 1. Raw material - Dexibuprofen 150.0g
[0059] 2. Solubilizing compound - sodium carbonate 75.0g
[0060] 3. Thinner - starch 20.0g
[0061] Dry starch 12.0g (pre-dried at 100℃~105℃)
[0062] 3. Adhesive - 10% starch slurry 12.0g
[0063] 4. Lubricant—magnesium stearate 1.2g
[0064]A total of 1000 tablets were produced, specification: 150mg (calculated as Dexibuprofen)
[0065] 2. Preparation method
[0066] The first step: pass the dextrobuprofen and sodium carbonate through a 80-mesh sieve respectively, mix them with starch,
[0067] Step 2: Add starch slurry to make soft material, granulate with a 14-mesh sieve, dry at 40°C-50°C, granulate at 12-mesh, add dry starch and magnesium stearate, mix well, and set aside.
[0068] The third step: the obtained material is tested by the ...
Embodiment 2
[0069] Embodiment 2 Preparation of Dexibuprofen pharmaceutical composition improving dissolution performance of the present invention
[0070] 1. Prescription B (orally disintegrating tablets)
[0071] 1. Raw material - Dexibuprofen 150.0g
[0072] 2. Solubilizing compound - arginine 122.0g
[0073] 3. Diluent - Mannitol 30.0g
[0074] 4. Disintegrant—cross-linked polyvinylpyrrolidone (PVPP) 50.0g
[0075] Microcrystalline Cellulose 28.0g
[0076] 5. Flavoring agent - Stevia 20.0g
[0077] Citric acid 10.0g
[0078] 6. Lubricant—magnesium stearate 2.0g
[0079] A total of 1000 tablets were produced, specification: 150mg (calculated as Dexibuprofen)
[0080] 2. Preparation method
[0081] The first step: pass the dextrobuprofen and auxiliary materials through a 100-mesh sieve respectively, and take by weighing.
[0082] Step 2: Take Dexibuprofen, mannitol, microcrystalline cellulose, citric acid and 2 / 3 prescription amount of cross-linked polyvi...
Embodiment 3
[0085] Embodiment 3 Preparation of Dexibuprofen Pharmaceutical Composition with Improved Dissolution Performance of the Present Invention
[0086] 1. Prescription C (capsule)
[0087] 1. Raw material - Dexibuprofen 150.0g
[0088] 2. Solubilizing compound - sodium carbonate 50.0g
[0089] Sodium bicarbonate 30.0g
[0090] 3. Thinner - starch 30.0g
[0091] Dry starch 12.0g (pre-dried at 100℃~105℃)
[0092] 4. Lubricant—magnesium stearate 1.5g
[0093] A total of 1000 tablets were produced, specification: 150mg (calculated as Dexibuprofen)
[0094] 2. Preparation method
[0095] The first step: pass the dextrobuprofen and auxiliary materials through a 60-mesh sieve respectively, and take by weighing.
[0096] Step 2: Mix well and set aside.
[0097] The fourth step: the obtained material is tested by the intermediate, and after the filling amount is determined, it is packed into capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com