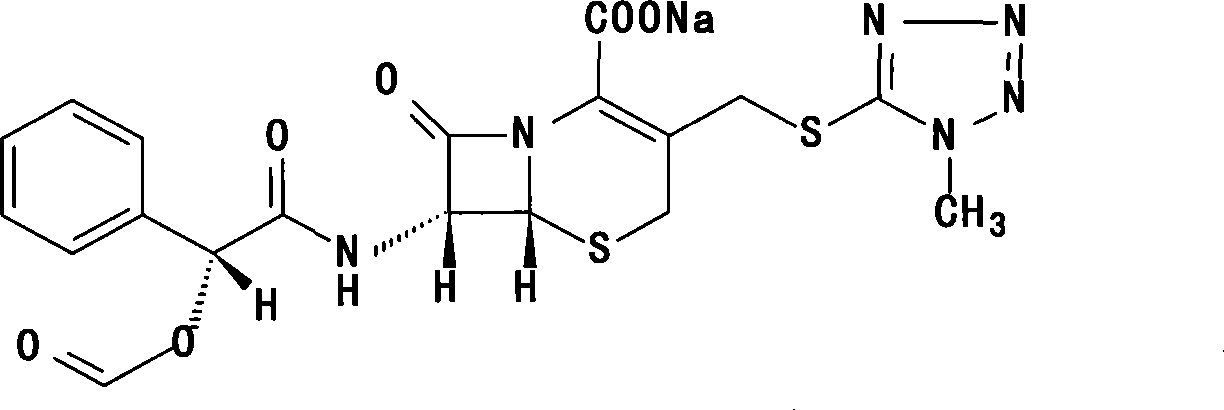
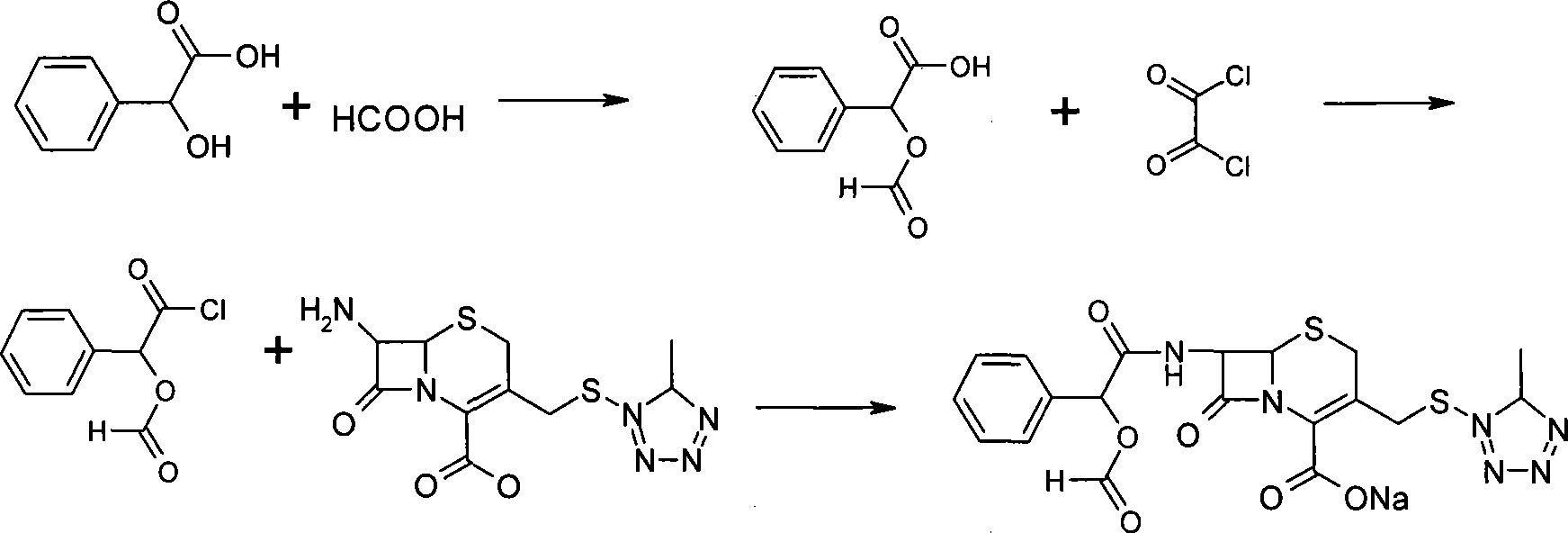
Cefamandole nafate powder injection, production method of powder injection and raw machine thereof
A technology of cefamandole sodium and sterile powder injection, which is applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of unsatisfactory product yield and purity and reaction conditions Harsh and other problems, to achieve good clarity, low cost of raw materials, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of Cefamandole Sodium Sterile Powder for Injection (Specification: 1.0 / bottle)
[0046] prescription:
[0047] Cefamandole Sodium 1000g
[0048] Anhydrous sodium carbonate 30g
[0049]
[0050] Total 1000 bottles
[0051] Preparation process: under sterile conditions, accurately weigh 1000g of cefamandole sodium and pulverize it, with a particle size of 90-120 μm, sieve, then weigh 30g of sterile sodium carbonate powder and place the sieved cefamandole sodium in the solid powder Mix evenly in the mixer, transfer the obtained raw materials to the aseptic preparation workshop, accurately measure and subpackage according to 1g of active ingredients per bottle, and press the cap to obtain the aseptic powder injection of cefamandole sodium.
Embodiment 2
[0053] Preparation of Cefamandole Sodium Sterile Powder for Injection (Specification: 1.0 / bottle)
[0054] prescription:
[0055] Cefamandole Sodium 1000g
[0056] Anhydrous sodium carbonate 25g
[0057]
[0058] Total 1000 bottles
[0059] Preparation process: under aseptic conditions, accurately weigh 1000g of cefamandole sodium and pulverize it, with a particle size of 75-90 μm, sieve, then weigh 25g of sterile sodium carbonate powder and place the sieved cefamandole sodium into the solid powder Mix evenly in the mixer, transfer the obtained raw materials to the aseptic preparation workshop, accurately measure and subpackage according to 1g of active ingredients per bottle, and press the cap to obtain the aseptic powder injection of cefamandole sodium.
Embodiment 3
[0061] Preparation of Cefamandole Sodium Sterile Powder for Injection (Specification: 1.0 / bottle)
[0062] prescription:
[0063] Cefamandole Sodium 1000g
[0064] Anhydrous sodium carbonate 30g
[0065]
[0066] Total 1000 bottles
[0067] Preparation process: under aseptic conditions, accurately weigh 1000g of cefamandole sodium and pulverize to a particle size of 75-90 μm, sieve, then weigh 30g of sterile sodium carbonate powder and place the sieved cefamandole sodium into the solid powder Mix evenly in the mixer, transfer the obtained raw materials to the aseptic preparation workshop, accurately measure and subpackage according to 1g of active ingredients per bottle, and press the cap to obtain the aseptic powder injection of cefamandole sodium.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com