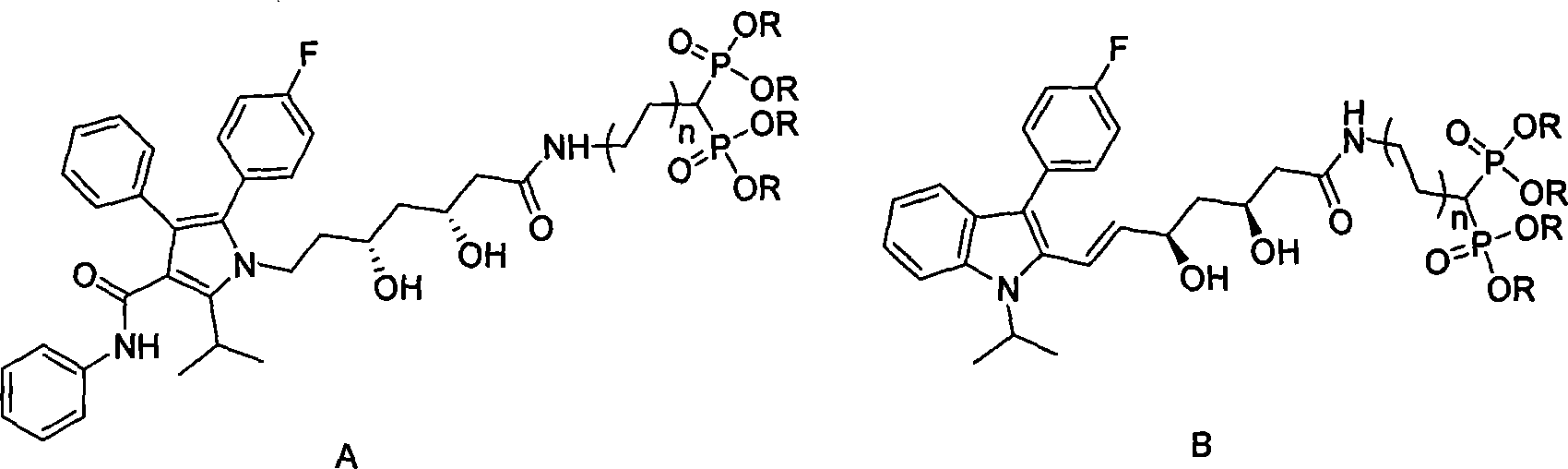
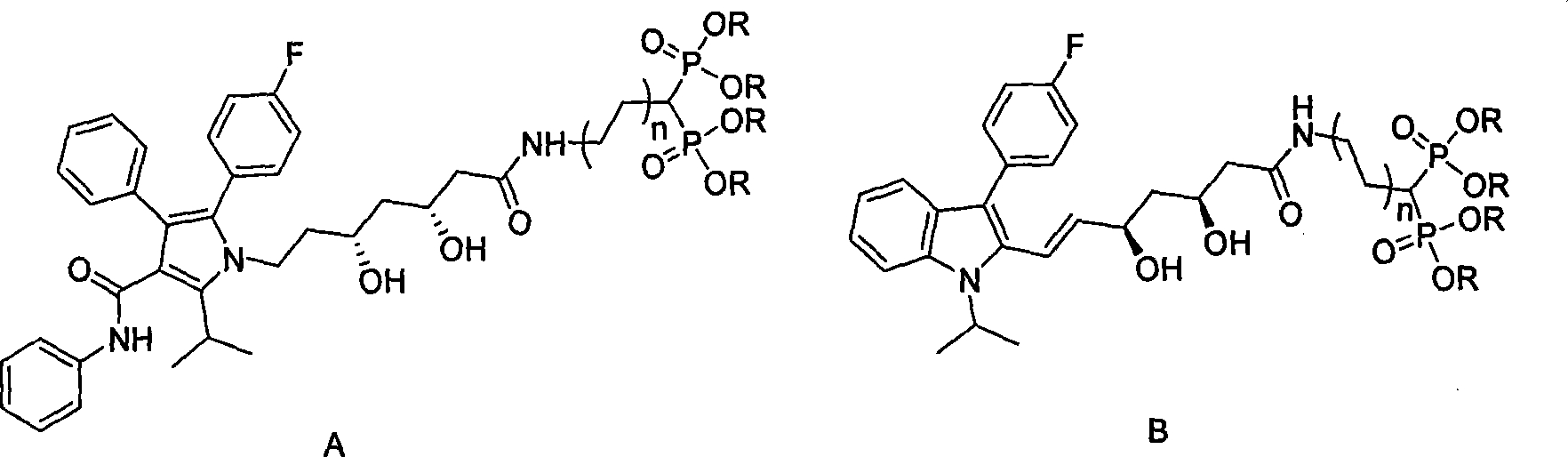
Statin-diphosphonic acid conjugates, preparation method and application thereof
A technology of a conjugate and bisphosphonate, applied in the field of statin-bisphosphonate conjugates, achieves the effects of good osteophilicity and a simple and feasible synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Preparation of fluvastatin lactone
[0022] Take 1228mg (2.98mmol) of fluvastatin, 484mg of N,N'-carbonyldiimidazole, dissolve in 100ml of dichloromethane, stir at room temperature for 48h, evaporate the solvent under reduced pressure, and use ethyl acetate:n-hexane (1:1) column Chromatography, the column chromatography product was dissolved in 5ml of ethyl acetate, and 30ml of petroleum ether was added dropwise to obtain 0.415g of fluvastatin lactone crystals, with a yield of 35.2%
[0023] (2) Statin-bisphosphonic acid conjugate ((3S, 5R, E)-7-(3-(4-fluorophenyl)-1-isopropyl-1-hydrogen-2-indole)-3, Preparation of 5-dihydroxy-6-heptenamide) tetraethyl methine diphosphate:
[0024] Get 166mg (0.4mmol) fluvastatin lactone, dissolve in 3ml (mass ratio 1: 15) tetrahydrofuran, add 183mg aminomethine bisphosphonate tetraethyl ester (0.6mmol, molar ratio 1.2 times), the reaction mixture is in Stir and react at 50°C for 48 hours. After the reaction is complete, distill o...
Embodiment 2
[0032] (1) Preparation of atorvastatin lactone:
[0033] Get atorvastatin 0.5g (0.813mmol), be dissolved in 10ml toluene, heat to reflux for 4 hours, evaporate solvent under reduced pressure, ethyl acetate:n-hexane:methanol=4:4:1 column chromatography, obtain atorvastatin Vastatin lactone 327mg. Yield 74.4%.
[0034] (2) 3-((3R, 5R)-7-(2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrole- Preparation of 1-yl)-3,5-dihydroxy-6-heptaneamide)propane-1,1-bisphosphonic acid tetraethyl ester
[0035] Take 327mg (0.605mmol) of atorvastatin lactone, 360mg (1.086mmol, molar ratio 1:3) of 3-aminopropane-1,1-bisphosphonate tetraethyl ester, dissolve in 20ml (mass ratio 1:53) Toluene, stirred at 70°C for 12 hours, after the reaction was complete, the solvent was distilled off under reduced pressure, and column chromatography of ethyl acetate:n-hexane:methanol (4:4:1) gave compound 5: 3-((3R,5R)-7- (2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1...
Embodiment 3
[0042] (1): 3-((3S,5R,E)-7-(3-(4-fluorophenyl)-1-isopropyl-1-hydrogen-2-indole)-3,5-dihydroxy Preparation of -6-heptenamide) propane-1,1-bisphosphate tetraethyl ester
[0043] Take 149mg (0.362mmol) of fluvastatin lactone, 240mg (0.706mmol, 2 times) of 3-aminopropane-1,1-bisphosphonate tetraethyl ester, dissolve in 7ml (mass ratio 1:41) benzene, 55°C Stirred for 20 hours, the reaction was complete, the solvent was distilled off under reduced pressure, and ethyl acetate:n-hexane:methanol (4:4:1) column chromatography gave compound 6,3-((3S,5R,E)-7-( 3-(4-Fluorophenyl)-1-isopropyl-1-hydrogen-2-indole)-3,5-dihydroxy-6-heptenamide)propane-1,1-bisphosphate tetraethyl Ester 0.211g, yield: 80.4% Compound NMR and phosphorus spectrum data are as follows:
[0044] 1 HNMR (CCl 3 D) 7.554-6.999 (m, 8H); 6.688 (d, 1H, J = 16.2); 5.709 (dd, 1H, J = 5.1, 15.6); 4.864 (t, 1H, J = 6.9); 4.480 (t, 1H, J=5.4); 4.230-4.119(m, 9H); 3.545-3.419(m, 2H); 2.462(tt, 1H, J=5.7, 24.6); 2.362-2.055(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com