Medicine gradient zero level implantation controlled-release drug administration device and preparation thereof
A drug delivery device and drug technology, applied in the direction of medical formula, drug delivery, medical preparations of non-active ingredients, etc., can solve the problems of insufficient research and development, and achieve simple preparation process, good reproducibility, and high degree of automation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the deployment of layer powder and binder
[0035] Pass polylactic acid through a 200-mesh sieve, and take powder with a particle size of less than 74 μm for the top and bottom layers; weigh 2 grams of polylactic acid powder, dissolve it in 50 mL of acetone, and prepare a polylactic acid containing 4% (w / v) The top and bottom powder forming binders.
[0036] The raw material composition and content (by weight percentage) of intermediate mixing powder are as follows:
[0037] Polylactic acid 100 parts
[0038] Colloidal silicon dioxide 2 parts
[0039] Macrogol 6000 20 parts
[0040] Weigh 13 grams of the drug chloramphenicol and dissolve in 100 mL of acetone to prepare an intermediate mixed powder forming binder.
Embodiment 2
[0041] Example 2: Determining 3D printing forming parameters
[0042] Top and bottom surface spray forming parameters:
[0043] Layer interval time 3min
[0044] Powder layer thickness 200μm
[0045] Spraying rate [spraying drop volume (droplet quantity × droplet size) × spraying frequency] 0.4nL × 12kz
[0046] Spray times 3 times
[0047] Parameters of intermediate medicated mixed powder spraying:
[0048] Layer interval time 5min
[0049] Powder layer thickness 200μm
[0050] Spraying rate [spraying drop volume (droplet quantity × droplet size) × spraying frequency] 0.4nL × 12kz
[0051] Spraying times 5 times
Embodiment 3
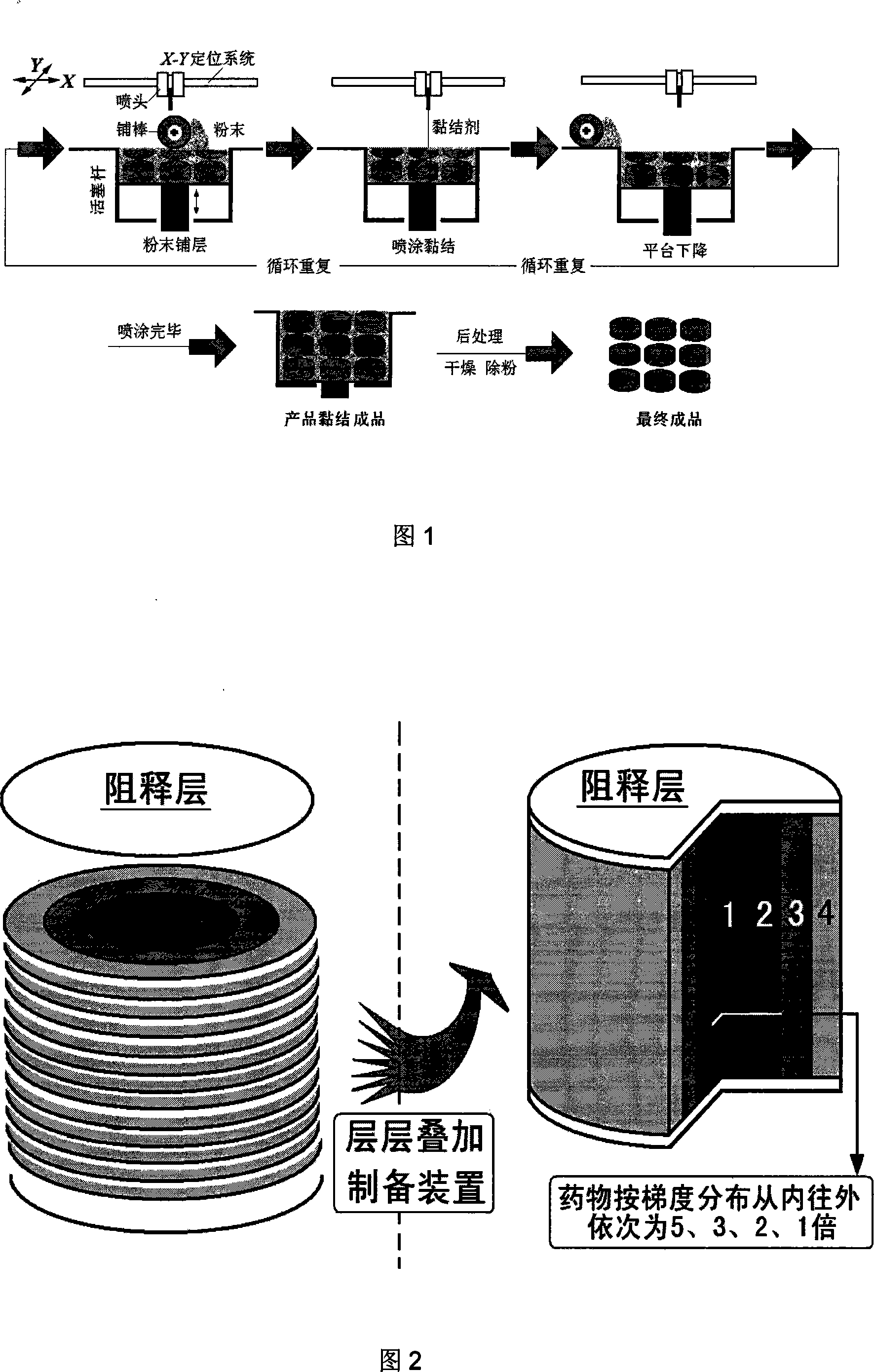
[0052] Example 3: Preparation of Drug Gradient Drug Delivery Device
[0053] The operation and preparation are directly controlled by the computer terminal output instructions. First lay a layer of polylactic acid powder with a thickness of 200 μm, spray 4% polylactic acid acetone solution three times as a binder to form the bottom surface of the drug delivery device, and then the piston rod drives the powder bed of the workbench to descend as a whole to prepare a new layer of polylactic acid powder. pink.
[0054] The middle layer is a mixed powder of polylactic acid and other auxiliary materials, the thickness of the layer is 200 μm, and the acetone solution containing 13% chloramphenicol is used as a binder. times, repeat 30 layers.
[0055] Subsequently, another layer of polylactic acid powder with a thickness of 200 μm was spread, and 4% (w / v) polylactic acid acetone solution was sprayed three times as a binder to form the top surface of the drug delivery device. Final...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com