Method for detecting residual solvent in medicine
A technology for residual solvents and drugs, applied in the field of detection of residual solvents in drugs, can solve problems such as qualitative determination of defects, and achieve the effects of simple assembly, easy promotion and saving test time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 The establishment of residual solvent database
[0037] Instruments and software:
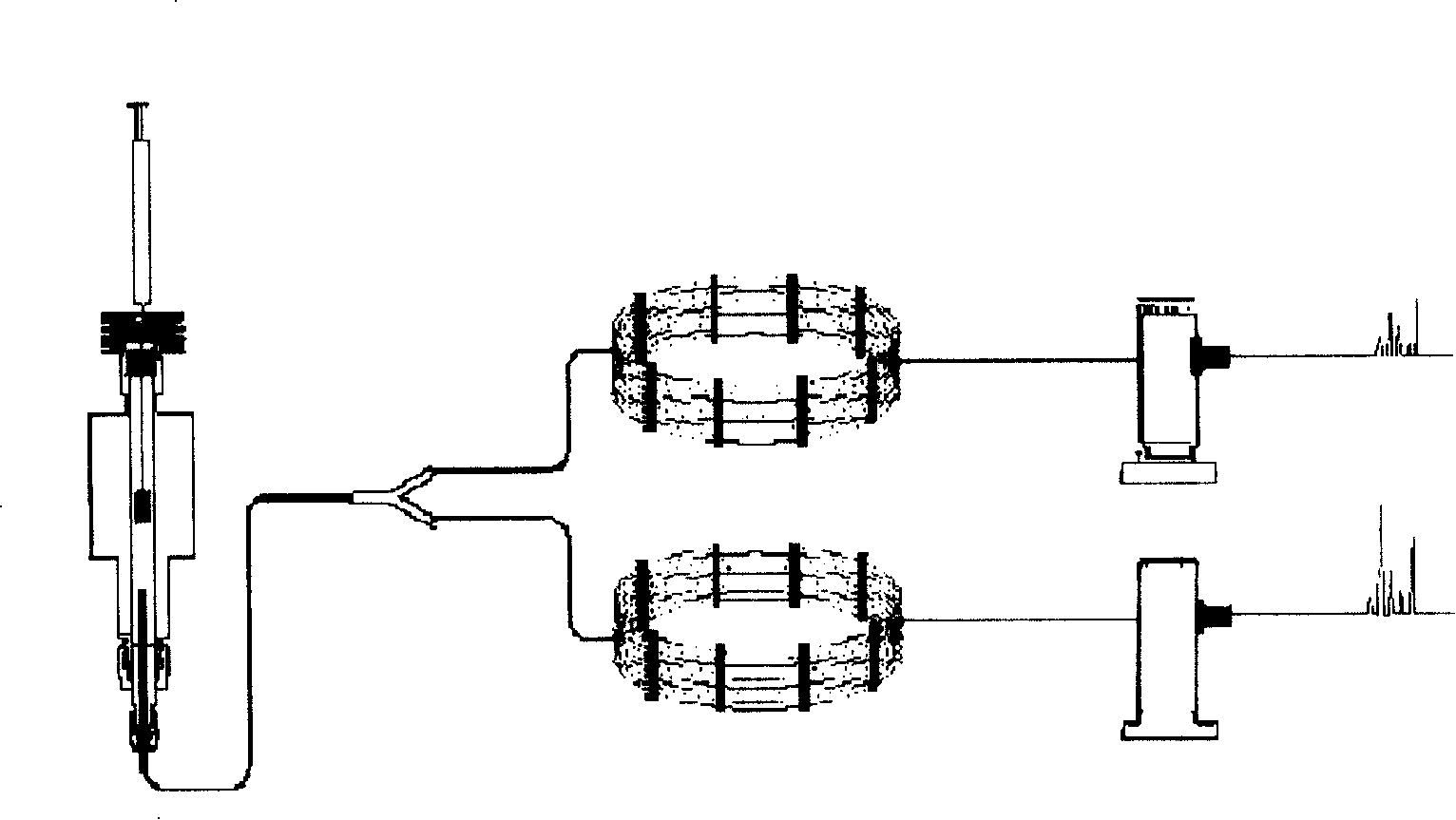
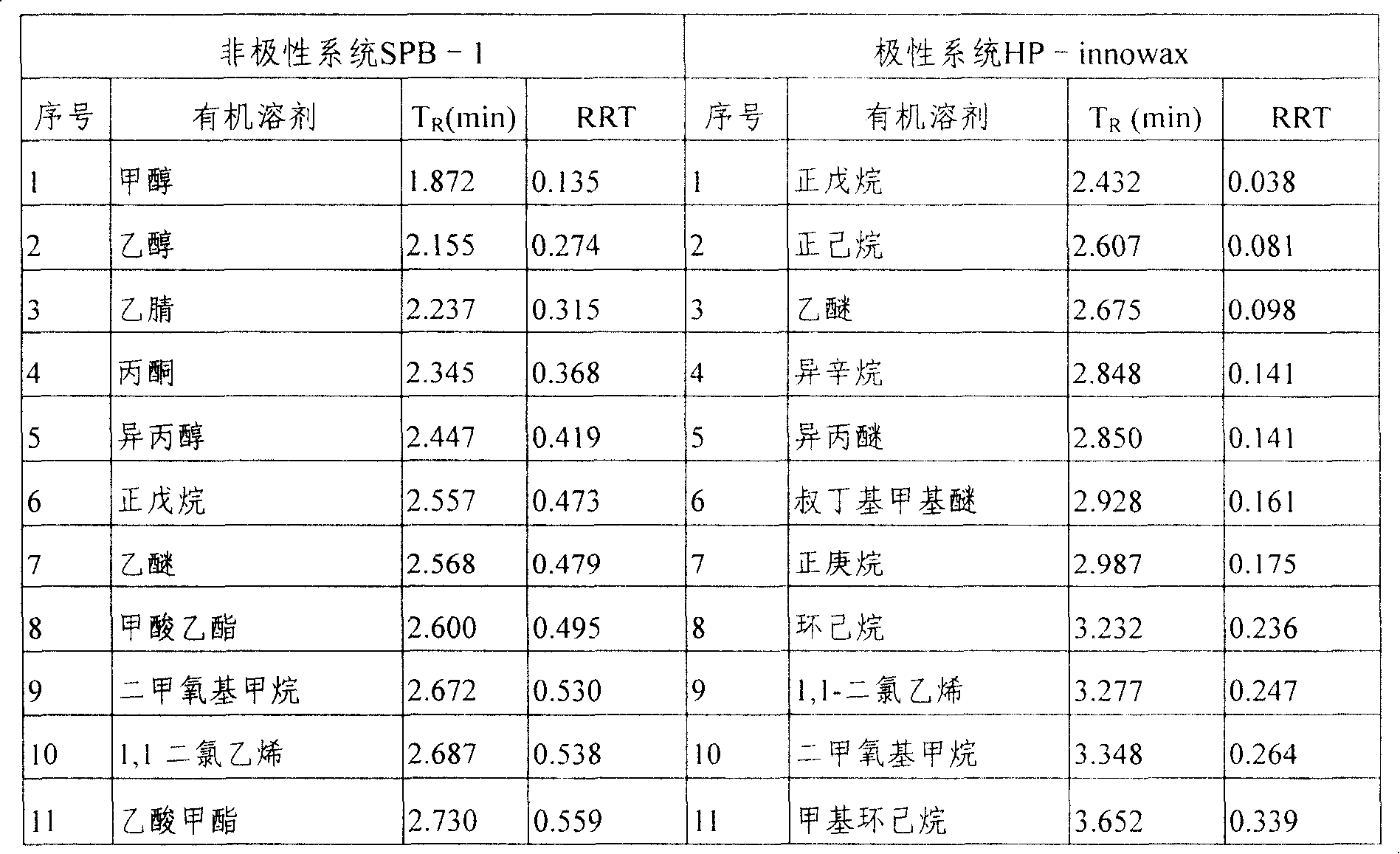
[0038] Trace GC Ultra (Thermo Electric Company); headspace system (Thermo Electric Company); figure 1 . Data analysis software is Xcalibur data processing system (Thermal Electric Company); Chromatographic column: SPB-1 type non-polar chromatographic column (30m × 0.32mm × 1um, Supelco company), HP-innowax type polar chromatographic column (30m × 0.32mm ×0.5um, Agilent)
[0039] Chromatographic conditions:
[0040]Temperature program: keep at 40°C for 8 minutes, then increase to 120°C at a rate of 8°C / min and hold for 10 minutes; inlet temperature: 200°C; constant flow rate: 4.0ml / min; carrier gas: helium; split ratio: 5 : 1; detector temperature: 250°C; headspace temperature: 70°C; headspace time: 20min.
[0041] Reagents: The reagents used were purchased from Aldrich Company, Fluka Company and Beijing Chemical Reagent Company, and the purity of all solvents was higher...
Embodiment 2
[0056] Determination of Residual Solvent in Example 2 Amoxicillin Sodium Clavulanate Potassium (5: 1)
[0057] The preparation of need testing solution: Accurately weigh 0.2384g of amoxicillin sodium clavulanate potassium (Korea Jingbao Pharmaceutical Co., Ltd., batch number D900400) (5:1), put in a 20ml headspace bottle, add 2.0ml water precisely, Seal the bottle tightly.
[0058] Preparation of butanone standard solution: Weigh 10 mg of butanone, put it in a 100ml volumetric flask, add water to the mark, measure 2ml, put it in a 20ml headspace bottle, and seal the mouth of the bottle.
[0059] Headspace condition and chromatographic condition are the same as embodiment 1, measure the retention time of butanone and the retention time of volatile components in need testing solution respectively, and calculate the chromatographic peak area, then compare with the database of embodiment 1 gained. See Table 3-1 and 3-2 for measurement results, Table 4-1 and 4-2 for content calcul...
Embodiment 3
[0075] Example 3 Determination of residual solvent in potassium clavulanate microcrystalline cellulose (1:1) The instruments and chromatographic conditions used are the same as those in Example 1.
[0076] Preparation of the test solution: accurately weigh 0.2139 g of potassium clavulanate microcrystalline cellulose (1:1), (Korea Jingbao Pharmaceutical Co., Ltd., batch number: D900400) put in a 20ml headspace bottle, and accurately add 2.0ml of water , Seal the mouth of the bottle.
[0077] Preparation of butanone standard solution: Weigh 10 mg of butanone, put it in a 100ml volumetric flask, add water to the mark, measure 2ml, put it in a 20ml headspace bottle, and seal the mouth of the bottle.
[0078] Headspace condition and chromatographic condition are the same as embodiment 2, measure the retention time of methyl ethyl ketone and the retention time of volatile component in need testing solution respectively, measure dead time with methane. The measurement results are sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com