Method and composition for increasing insulin sensibility
A technology of insulin sensitivity and composition, applied in the fields of biotechnology and pharmacy, can solve problems such as vomiting, dehydration, and accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] Example 1 SIRT1 protein levels are reduced in the case of insulin resistance
[0161] 1. Using C2C12 as a cell model
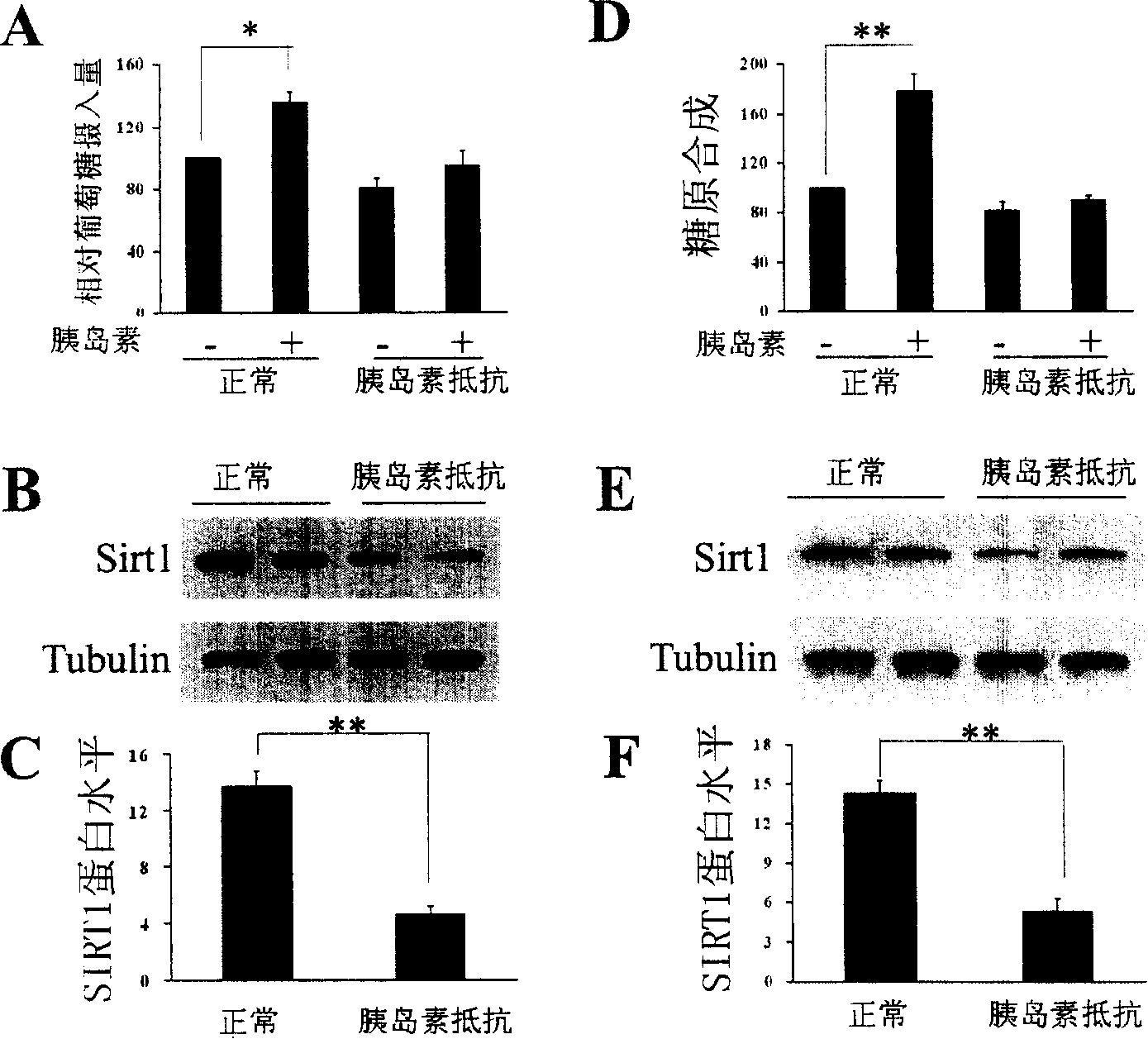
[0162] According to the aforementioned method, C2C12 cells induced by palmitic acid to produce insulin resistance were prepared, and the expression of SIRT1 protein in the cells was determined. see results figure 1 A- figure 1 c.
[0163] figure 1 A is the measurement of glucose transport (relative glucose intake) of C2C12 cells by the aforementioned glucose transport assay method. The results show that C2C12 cells produce insulin resistance after being induced by palmitic acid (that is, in the absence and presence of insulin, relative Glucose intake did not change significantly).
[0164] figure 1 B is the result of measuring the expression of SIRT1 protein by the aforementioned Western Blot method, and the SIRT1 protein decreases in insulin-resistant C2C12 cells; figure 1 C is figure 1 Quantitative representation of B. In the figure, tubulin ...
Embodiment 2
[0171] Example 2 Down-regulation of SIRT1 can lead to insulin resistance
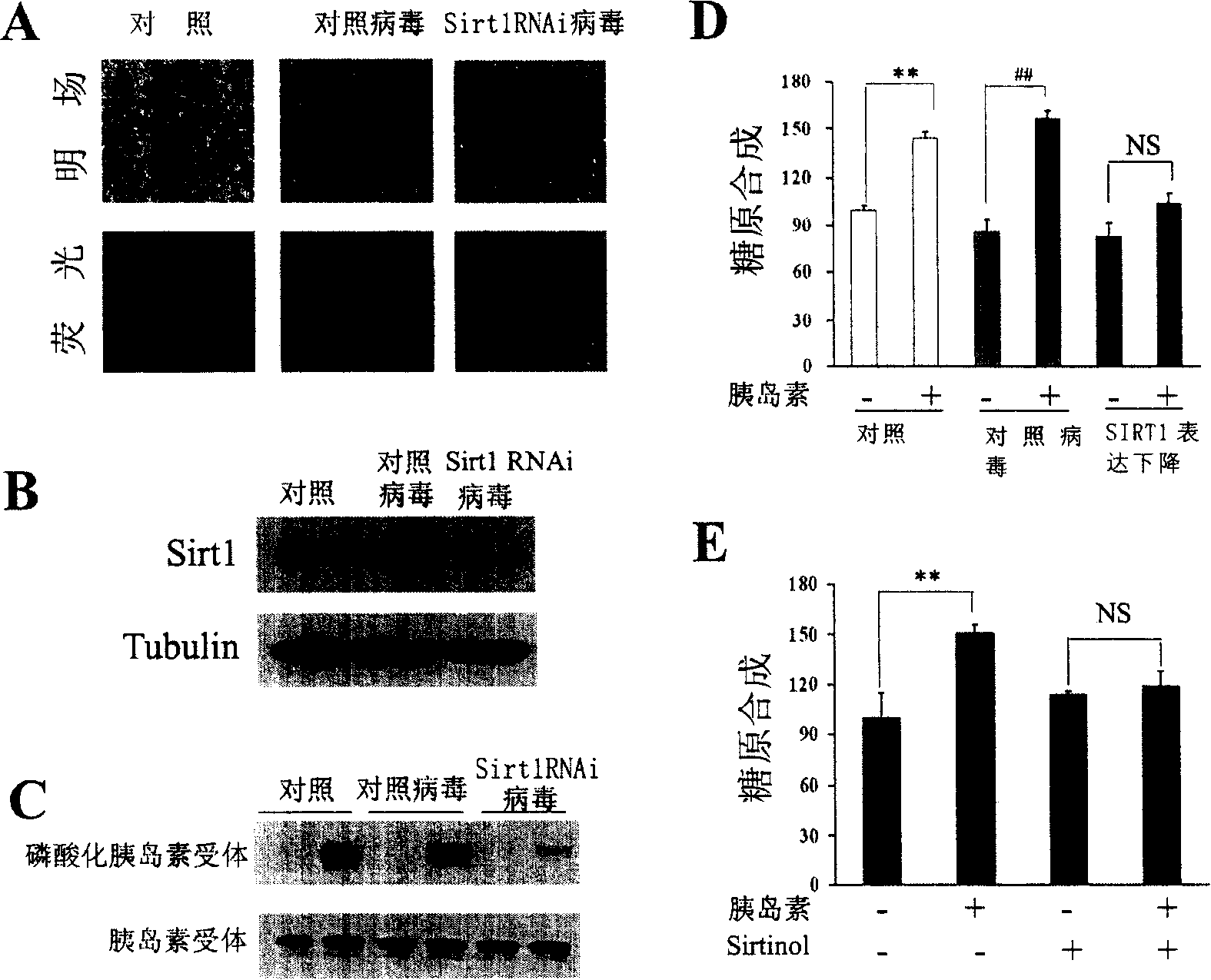
[0172] According to the aforementioned method, HepG2 cells were infected with the lentivirus prepared above, and RNA interference was performed on SIRT1 in the cells, thereby resulting in a decrease in the expression of SIRT1 protein.
[0173] HepG2 cells were infected with lentivirus for 72 hours, and both the control virus and SIRT1 RNA interference virus could express fluorescent protein, so the infection efficiency of the lentivirus used was judged by observing the expression of fluorescent protein. Fluorescence photos of HepG2 cells infected with lentivirus as shown in figure 2 A, the results show that the efficiency of lentivirus infection of HepG2 cells is very high, and the control virus is Luc RNA interference.
[0174] figure 2 B is the result of Western Blot detection of intracellular SIRT1 protein after RNA interference. Depend on figure 2 B shows that lentivirus-mediated RNA interfer...
Embodiment 3
[0179] Example 3 Effects of Up-regulation of SIRT1 Protein Level on Glucose Intake and Insulin Activation Signal under Normal and Insulin Resistance Conditions
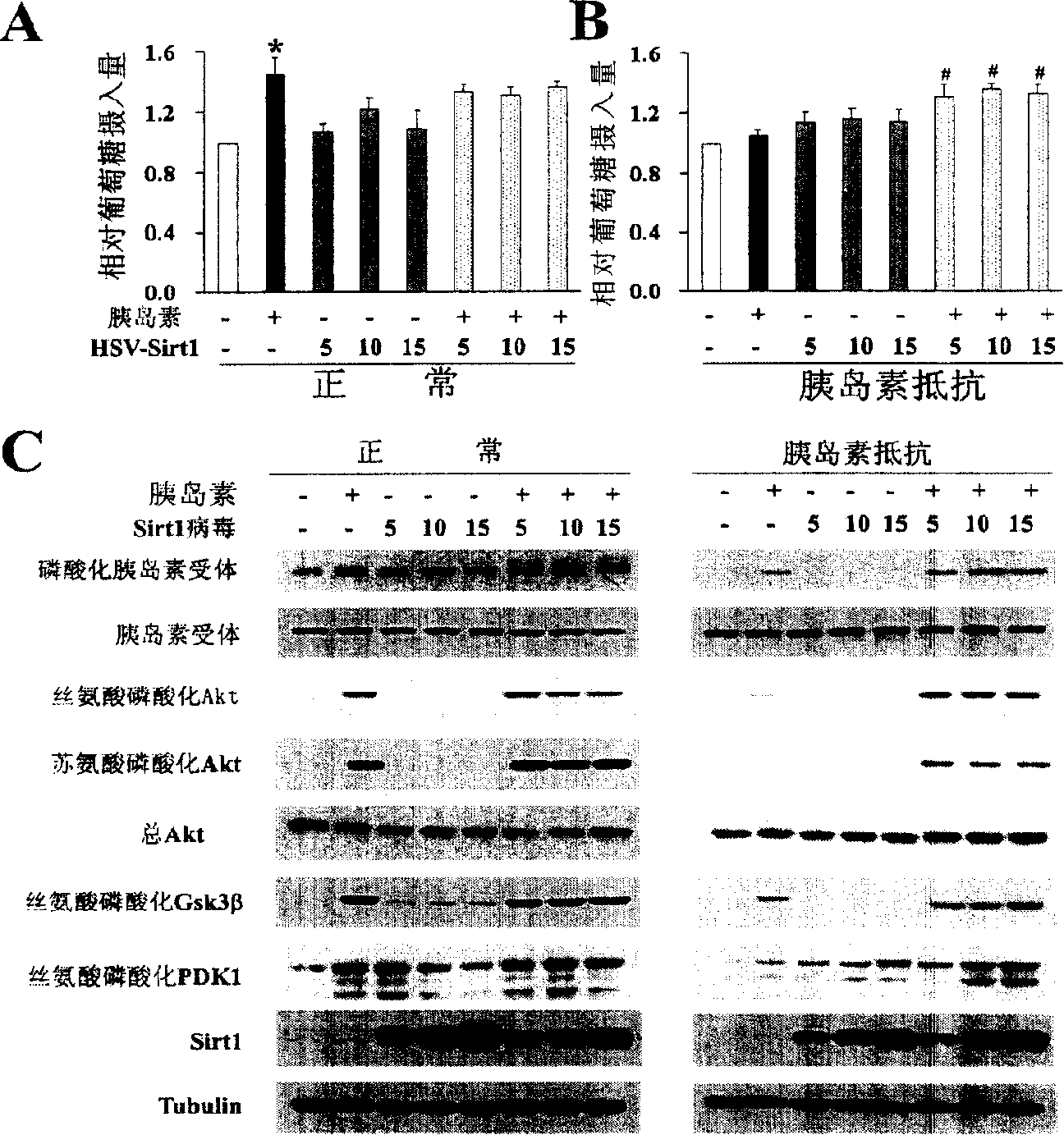
[0180] The virus HSV-SIRT1 was prepared according to the aforementioned method, and C2C12 cells were treated with HSV-SIRT1 to up-regulate the protein level of SIRT1 in C2C12 cells, and its effects on glucose intake and insulin activation signals in normal and insulin resistant conditions were determined. The result is as image 3 A- image 3 C shown.
[0181] Depend on image 3 A shows that under normal circumstances, up-regulation of SIRT1 protein level has no significant effect on glucose intake. In the figure, the white column represents the group without insulin and HSV-SIRT1, the black column represents the group with insulin alone, the gray column represents the group with HSV-SIRT1 alone, and the column with black dots and white bottom represents the group with insulin and HSV-SIRT1 .
[0182] Depend on ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com