RBP4 antibody and its preparation method and use
A polyclonal antibody and monoclonal antibody technology, applied in the field of retinol-binding protein, can solve the problems of inability to meet the sensitivity requirements, difficult for antibodies to detect natural proteins, and unsatisfactory detection effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
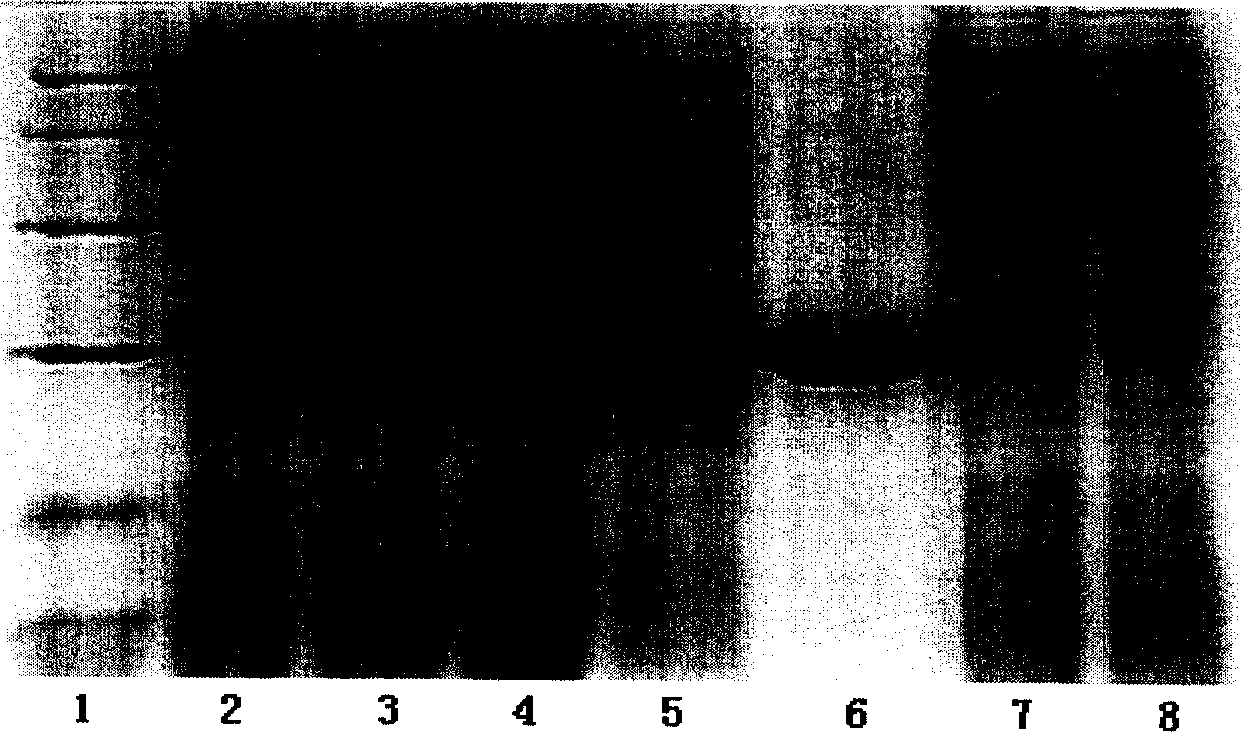
[0060] Example 1: Prokaryotic protein expression
[0061] The present inventors extracted mRNA from human liver tissue, used GGCGGATTCCTGGGCAAGAT (SEQ ID NO: 1) as a forward primer, and GATGGGGAGAGAAGGGCAAA (SEQ ID NO: 2) as a reverse primer, and obtained the RBP4 gene by RT-PCR. The cloned gene was verified by sequencing to contain no meaningful mutations. By adding primers with BamH I and Xho I restriction sites at both ends (with CGCGGATCCATGAAGTGGGTGTGGGCGCTCTT (SEQ ID NO: 3) as the forward primer and CCGCTCGAGGCTACAAAAGGTTTCTTTCTGATC (SEQ ID NO: 4) as the reverse primer), the inventors will clone the The gene was connected to the BamH I and Xho I sites of the pET28a vector to obtain the pET28a-RBP4 recombinant plasmid.
[0062] Afterwards, the recombinant plasmid was transformed into BL21 expressing Escherichia coli, induced under the condition of 0.5mM IPTG, and the expressed protein mainly existed in the inclusion body. The RBP4 protein expressed in the inclusion body...
Embodiment 2
[0064] Example 2: Animal immunization
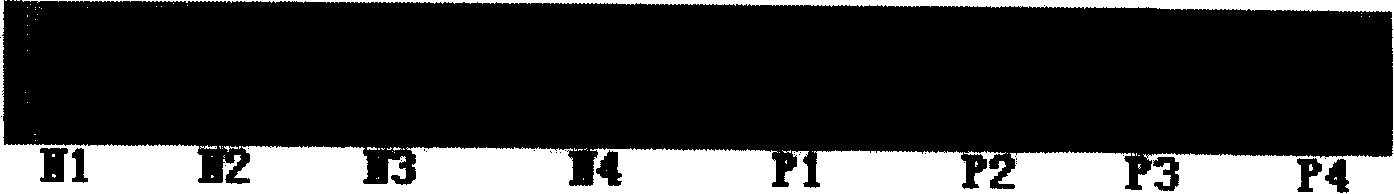
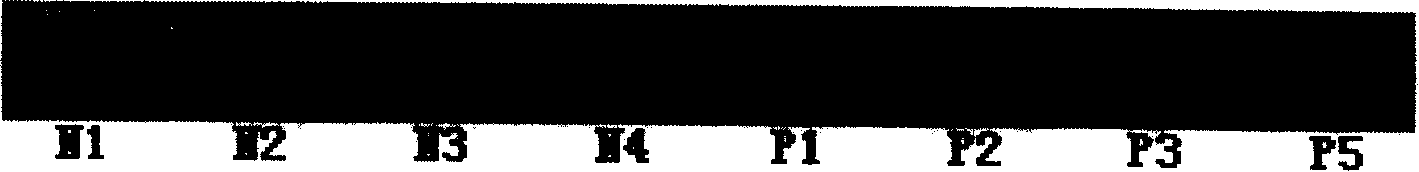
[0065] Mice were immunized with RBP4 purified in Example 1. Balb / c mouse immunization dose: 0.1 mg each time. Multiple intramuscular injections. Immunization program: 0, 3, 6 three times immunization. Three days before the fusion, 0.1 mg protein was injected intraperitoneally for memory stimulation.
[0066] Rabbits were immunized with RBP4 purified in Example 1. Immune dose of big-eared rabbits: 0.5mg each time. Multiple intramuscular injections. Immunization program: 0, 3, 6 three times immunization. Blood was collected after titer assessment.
Embodiment 3
[0067] Example 3: Construction of hybridoma cell lines and preparation of monoclonal antibodies
[0068] Fusion was done three days after recall stimulation. The spleen cells of the immunized mice were fused with the mouse myeloma cell SP2 / 0 by conventional fusion method of polyethylene glycol (PEG).
[0069] After fusion, HAT selective medium was added, and ELISA was used for screening, and the antigen used for screening was the purified RBP4 protein in Example 1.
[0070] A monoclonal antibody was obtained after cloning and screening by limiting dilution three times, named S-18-16.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com