Method for synthesizing (4S,5R)- half-ester
A synthetic method, cinchona technology, applied in the synthesis field of -half-ester, can solve the problems such as troublesome catalyst preparation, achieve high enantioselective catalytic effect, high chemical yield and optical purity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
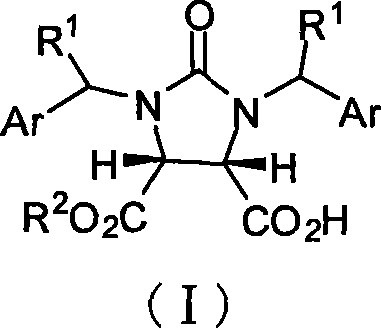
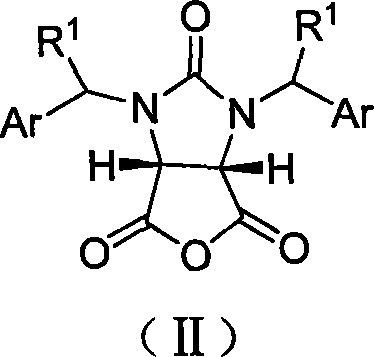
[0027] Example 1 cis-1,3-dibenzylimidazolin-2-one-2H-furo[3,4-d]imidazole-2,4,6-trione (33.6g, 0.10mol), 9 -propargylquinine (32.4g, 0.10mol), carbon tetrachloride (600mL) and toluene (400mL) were placed in a dry reaction flask, and anhydrous methanol (40.5mL, 1.0mol) was added dropwise at -40°C, After the addition was complete, the reaction was stirred at -40°C for 60 hours. After the reaction was complete, the solvent was recovered under reduced pressure, cooled to room temperature, ethyl acetate (500 mL) was added to the residue and stirred for 15 min, then 2M hydrochloric acid (400 mL) was added and stirred at 10-15°C for 10 min, left to stand, and the organic layer was separated. water and sodium sulfate to dry. Filtration, the filtrate reclaims the solvent under reduced pressure, and the residue is recrystallized with benzene (150mL) to obtain a white crystalline powder I (R 1 =-H, Ar =-Ph, R 2 =-CH 3, 35g, 95%), mp149~150℃, [α] D 22 =+2.74° (c 0.20, CHCl 3 ). IR...
Embodiment 2
[0031] Example 2 cis-1,3-dibenzylimidazolin-2-one-2H-furo[3,4-d]imidazole-2,4,6-trione (33.6g, 0.10mol), 9 - propargyl quinine (32.4g, 0.10mol), carbon tetrachloride (600mL) and toluene (400mL) were placed in a dry reaction flask, and cyclohexanol (52.2mL, 0.50mol) was added dropwise at -40°C, After the addition was complete, the reaction was stirred at -40°C for 60 hours. After the reaction was complete, the solvent was recovered under reduced pressure, cooled to room temperature, ethyl acetate (500 mL) was added to the residue and stirred for 15 min, then 2M hydrochloric acid (400 mL) was added and stirred at 10-15 ° C for 15 min, and the organic layer was separated. water and sodium sulfate to dry. Filtration, the filtrate reclaims the solvent under reduced pressure, and the residue is recrystallized with benzene (160mL) to obtain a white crystalline powder I (R 1 -H, Ar = -Ph, R 2 =cyclohexyl, 37.4g, 87%), mp172~175°C, [α] D 22 =+2.70° (c 0.20, CHCl 3 ), [α] D 25 =...
Embodiment 3
[0032] Example 3 cis-1,3-dibenzylimidazolin-2-one-2H-furo[3,4-d]imidazole-2,4,6-trione (33.6g, 0.10mol), 9 - Propargyl dihydroquinine (32.6g, 0.10mol), carbon tetrachloride (600mL) and toluene (400mL) were placed in a dry reaction flask, and anhydrous methanol (40.5mL, 1.0mol ), the reaction was stirred at -40°C for 60 hours after addition. After the reaction was complete, the solvent was recovered under reduced pressure, cooled to room temperature, ethyl acetate (500 mL) was added to the residue and stirred for 15 min, then 2M hydrochloric acid (400 mL) was added and stirred at 10-15°C for 10 min, left to stand, and the organic layer was separated. water and sodium sulfate to dry. Filtration, the filtrate reclaims the solvent under reduced pressure, and the residue is recrystallized with toluene (200mL) to obtain a white crystalline powder I (R 1 -H, Ar = -Ph, R 2 =-CH 3 , 33.2g, 90%), mp148~150℃, [α] D 22 =+2.70° (c 0.20, CHCl 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com