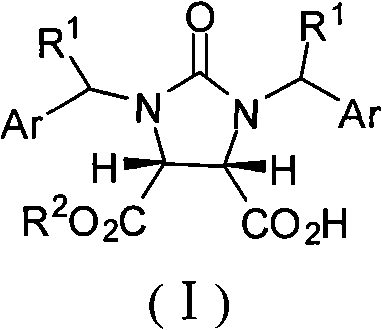
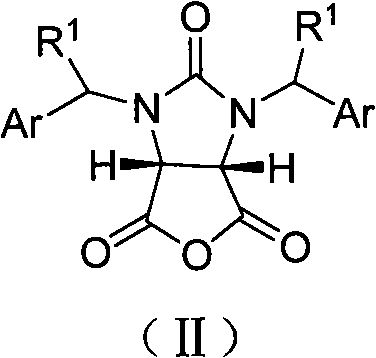
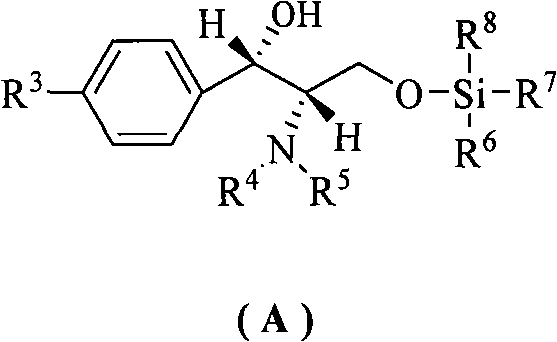
Synthetic method of (4S,5R)-half-ester
A synthesis method and technology of alkyl, applied in the field of synthesis of beta-half esters, can solve the problems of high price, difficult industrial production, and difficulty in catalyst preparation, and achieve easy operation, easy preparation, recyclable chemical yield and optical purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: cis-1,3-dibenzyl-tetrahydro-2H-thieno[3,4-d]imidazole-2,4,6-trione (33.6g, 0.10mol), methanol (8.1 mL, 0.20mol), (1S,2S)-1-(4-nitrophenyl)-2-(N,N-dimethylamino)-3-tert-butyldimethylsilyloxy-1- Propanol (10.6g, 0.03mol) and tetrahydrofuran (200mL) were dried and placed in a reaction flask, and stirred at -15~-10°C for 36h. After the reaction was complete, the solvent was recovered under reduced pressure, cooled to room temperature, ethyl acetate (140 mL) was added to the residue and stirred for 15 min, then 5% hydrochloric acid (400 mL) was added and stirred at 10-15°C for 10 min, and the organic layer was separated. Dry over anhydrous sodium sulfate. Filtrate, and recover the solvent from the filtrate under reduced pressure, add toluene (35mol), stir for 15min, a solid is precipitated, and dried to obtain a white powder III (R=CH 3 , 35g, 95%), mp148~150℃, [α] D 22 =+2.73° (c 0.20, CHCl 3 ), IR (KBr): ν=2979, 2384, 2280, 1742, 1462, 1229, 1169, 768cm -1...
Embodiment 2
[0027] Example 2: cis-1,3-dibenzyl-tetrahydro-2H-thieno[3,4-d]imidazole-2,4,6-trione (33.6g, 0.10mol), benzyl alcohol ( 41g, 0.38mol), (1S, 2S)-1-(4-nitrophenyl)-2-(N,N-diethylamino)-3-O-trimethylsilyl-1-propanol (15.6 g, 0.05mol), tetrahydrofuran (300mL) and toluene (200mL) were placed in a dry reaction flask, and stirred at -10~-5°C for 45h. After the reaction was complete, the solvent was recovered under reduced pressure, cooled to room temperature, ethyl acetate (150 mL) was added to the residue, stirred for 10 min, then 20% acetic acid (300 mL) was added, stirred at 10-15°C for 5 min, left to stand, and the organic layer, dried over anhydrous sodium sulfate. Filtrate, recover the solvent from the filtrate under reduced pressure, add toluene (350mL), stir for 20min, a solid precipitates, and dry to obtain a white powder III (R=PhCH 2 , 37.8g, 85%), mp 57.7~60.6℃, [α] D 25 =+12.2° (c 0.20, CHCl 3 ). IR(KBr): ν=3031, 2945, 1752, 1664, 1454, 1236, 1202, 967, 741, 700cm ...
Embodiment 3
[0028] Example 3: cis-1,3-dibenzyl-tetrahydro-2H-thieno[3,4-d]imidazole-2,4,6-trione (33.6g, 0.10mol), methanol (14.2 mL, 0.35mol), (1S, 2S)-1-(4-nitrophenyl)-2-(N-methyl-N-benzyl)-3-O-tert-butyldisilyl (3.89 g, 0.01mol), carbon tetrachloride (300mL) and toluene (200mL) were placed in a dry reaction flask, and stirred at -25~-20°C for 50h. After the reaction was complete, the solvent was recovered under reduced pressure, cooled to room temperature, ethyl acetate (250 mL) was added to the residue, stirred for 5 min, then 20% sulfuric acid (300 mL) was added, stirred at 15° C. for 10 min, and the organic layer was separated. Dry over anhydrous sodium sulfate. Filtrate, the filtrate recovered the solvent under reduced pressure, added toluene (350mL), stirred for 5min, a solid precipitated, dried to obtain a white powder III (R=CH 3 , 29g, 90%), mp 147~150℃, [α] D 22 =+2.70° (c 0.20, CHCl 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com