Method for mass preparation of formamide phenol compound
A large-scale preparation technology of retinoic acid, applied in the direction of organic chemistry, etc., can solve the problems of increasing the protection and deprotection process, high requirements for reaction equipment, cumbersome routes, etc., and achieve cheap reaction reagents and short reaction steps , good effect of reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
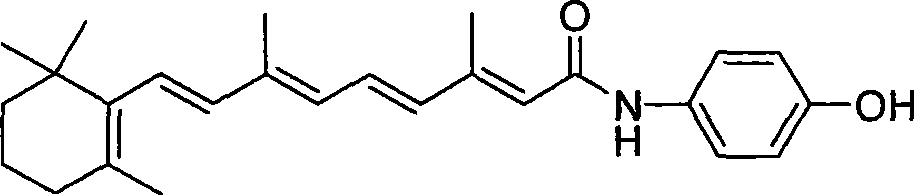
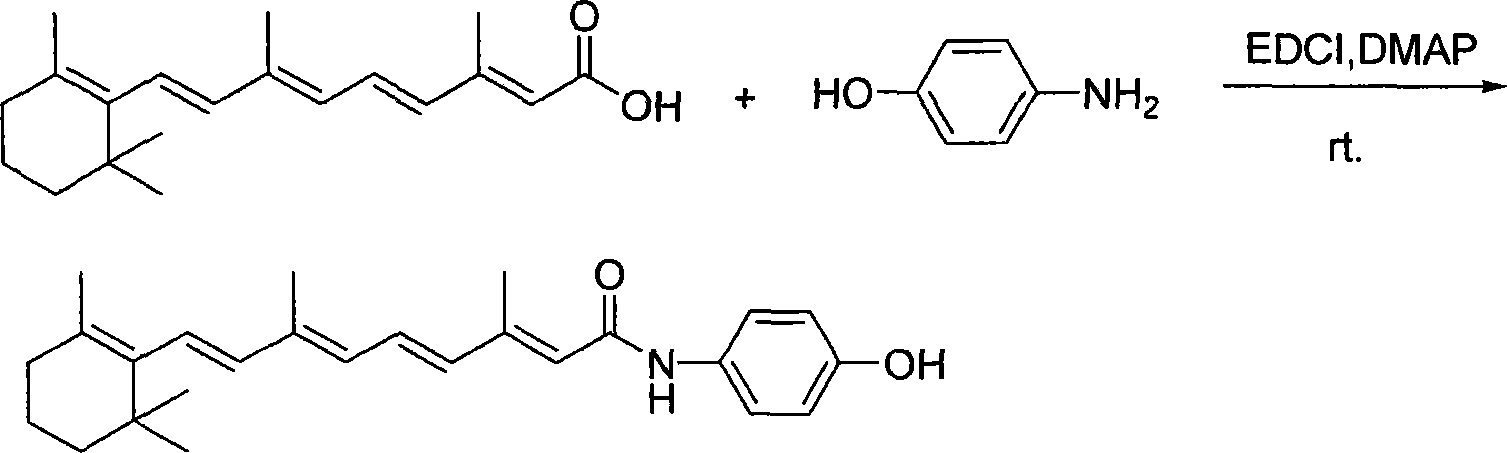
[0029] Add 47.3ml of thionyl chloride (SOCl 2 , 0.666mol) and 51.3ml of anhydrous N,N-dimethylformamide (DMF, 0.666mol), stirred under nitrogen protection for 1h. Add 500 ml of anhydrous DMF containing 100 g of all-trans retinoic acid (0.333 mol) and react for 3 h at 0° C. in the dark to obtain a red acid chloride solution. Then add 92.3ml triethylamine (Et 3 N, 0.666mol), and continued to react for 1 h to obtain a deep red acyl ammonium salt solution.
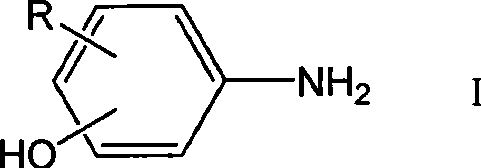
[0030] Add 108.0g p-aminophenol (0.99mol) in 2 liters of three-necked flask equipped with thermometer, dropping funnel and drying tube, dissolve with 500ml anhydrous DMF, then add dropwise the above-mentioned acid chloride solution, the temperature is controlled at Below 20°C, after the dropwise addition, continue to stir until the reaction is complete as monitored by thin layer chromatography (TLC). Pour the reaction solution into 5 L of NH 4 Cl saturated aqueous solution, extracted with ethyl acetate, and then the organi...
Embodiment 2
[0034] Add 61.2ml of phosphorus oxychloride (POCl 3 , 0.666mol) and 51.3ml DMF (0.666mol), stirred under nitrogen protection for 1h. Add 500 ml of anhydrous DMF containing 100 g of all-trans retinoic acid (0.333 mol) and react for 3 h at 0° C. in the dark to obtain a red acid chloride solution. Then add 92.3ml Et 3 N (, 0.666mol), the reaction was continued for 1h to obtain a deep red acyl ammonium salt solution.
[0035] Add 108.0g p-aminophenol (0.99mol) in 2 liters of three-necked flask equipped with thermometer, dropping funnel and drying tube, dissolve with 500ml anhydrous DMF, then add dropwise the above-mentioned acid chloride solution, the temperature is controlled at Below 20°C, after the dropwise addition, continue to stir until the reaction is complete as monitored by TLC. Pour the reaction solution into 5 L of NH 4 Cl saturated aqueous solution, extracted with ethyl acetate, and then the organic layer was washed with water, saturated brine, Na 2 SO 4 Dry over...
Embodiment 3
[0037] Add 47.3ml SOCl to a 1-liter eggplant-shaped bottle 2 (0.666mol) and 51.3ml DMF (0.666mol), stirred under nitrogen protection for 1h. Add 500 ml of anhydrous DMF containing 100 g of all-trans retinoic acid (0.333 mol) and react for 3 h at 0° C. in the dark to obtain a red acid chloride solution. Then 53.8 ml of pyridine (Py, 0.666 mol) was added, and the reaction was continued for 1 h to obtain a deep red acyl ammonium salt solution.
[0038]Add 108.0g p-aminophenol (0.99mol) in 2 liters of three-necked flask equipped with thermometer, dropping funnel and drying tube, dissolve with 500ml anhydrous DMF, then add dropwise the above-mentioned acid chloride solution, the temperature is controlled at Below 20°C, after the dropwise addition, continue to stir until the reaction is complete as monitored by TLC. Pour the reaction solution into 5 L of NH 4 Cl saturated aqueous solution, extracted with ethyl acetate, and then the organic layer was washed with water, saturated b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com