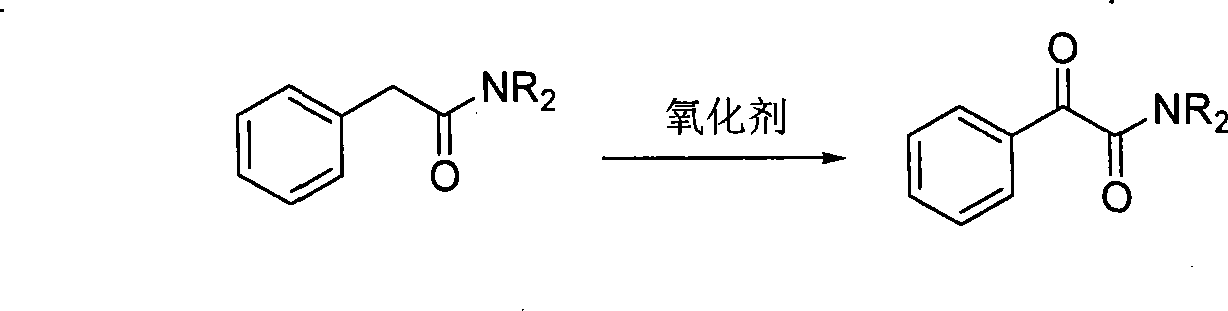
Synthesis method for alpha-carbonylamide compound
A technology of carbonyl amides and synthesis methods, which is applied in the field of synthesis of N-substituted α-carbonyl amides, can solve the problems of high toxicity of reaction raw materials or oxidants, difficulty in obtaining raw materials, harsh reaction conditions, etc., and achieve mild conditions and easy raw materials. Get and respond to the effect of high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of 1-phenyl-2-piperidinyl-1,2-ethyldione
[0028] The preparation of 1-phenyl-2-piperidinyl-1,2-ethyl diketone adopts the following steps: 1. add 10 gram N-piperidinyl phenylacetamides, 1.6 gram cesium carbonate in 250 ml round bottom flask , 1.6 grams of tetrabutylammonium bromide, 100 milliliters of DMF, put in a drying tube and heat to 120 ° C, follow the reaction with thin layer chromatography until the reaction raw material N-piperidinyl phenylacetamide disappears; An appropriate amount of ethyl acetate was added to the system to dilute the reaction, and then the alkali and additives were filtered out. The crude product was obtained after removing the solvent in the filtrate with a rotary evaporator; ③ the crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate=5: 1) to obtain 9.9 grams of light yellow solid, namely 1-phenyl- 2-Piperidinyl-1,2-ethyldione, 93% yield. Melting point: 104-105°C.
[0029] ...
Embodiment 2
[0033] Example 2: Preparation of 1-p-methoxyphenyl-2-piperidinyl-1,2-ethyldione
[0034] The preparation of 1-p-methoxyphenyl-2-piperidinyl-1,2-ethyldiketone adopts the following steps: 1. add 7 grams of N-piperidinyl-p-methoxyl in a 250 ml round bottom flask Phenylphenylacetamide, 1.2 grams of cesium carbonate, 3.3 grams of tetrabutylammonium bisulfate, 100 milliliters of DMF, heated to 120 ° C after installing a drying tube, followed the reaction by thin layer chromatography, until the reaction raw material N-piperidinyl- p-methoxyphenylacetamide disappeared; ②After the reaction, add an appropriate amount of ethyl acetate to the system to dilute the reaction, and then filter out the alkali and additives. The crude product was obtained after removing the solvent in the filtrate with a rotary evaporator; ③ the crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 5: 1) to obtain 6.8 grams of light yellow liquid, which was 1-p-methoxy ...
Embodiment 3
[0039] Example 3: Preparation of 1-p-nitrophenyl-2-piperidinyl-1,2-ethyldione
[0040] The preparation of 1-p-nitrophenyl-2-piperidinyl-1,2-ethyldiketone adopts the following steps: 1. add 7 grams of N-piperidinyl-p-nitrobenzene in a 250 ml round bottom flask Acetamide, 1.9 grams of cesium carbonate, 1.8 grams of tetrabutylammonium iodide, 100 milliliters of DMF, put in a drying tube and heat to 100-120 ° C, follow the reaction by thin layer chromatography until the reaction raw material N-piperidinyl- p-Nitrophenylacetamide disappears; ②After the reaction, add an appropriate amount of ethyl acetate to the system to dilute the reaction, and then filter out the alkali and additives. The crude product was obtained after removing the solvent in the filtrate with a rotary evaporator; ③ the crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 3: 1) to obtain 4.8 grams of a light yellow solid, which was 1-p-nitro Phenyl-2-piperidinyl-1,2-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com