Sulbactam sodium bacteriophage complex and the preparing method thereof
A technology of sulbactam sodium and preparation process, which is applied in the field of sulbactam sodium antibiotic compound and its preparation process, and can solve the problems of relatively large difference in elimination half-life between antibiotics and sulbactam sodium, kidney damage, and easy oxidation of sulbactam sodium To achieve the effect of prolonging the elimination half-life, reducing toxic effects, and reducing allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
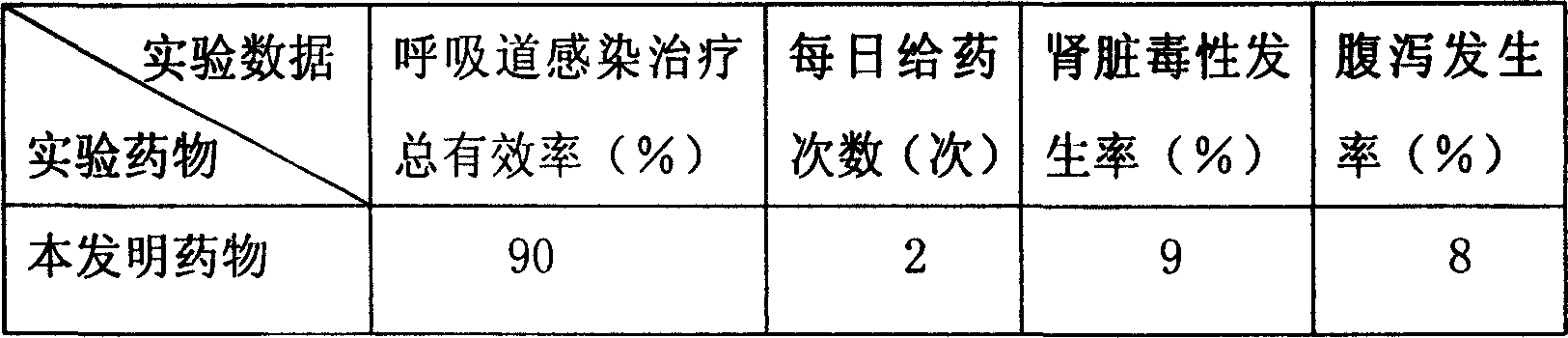
[0043] The present embodiment adopts the mouse of respiratory tract infection disease model as experimental animal, carries out comparative experiment with the mixture of sulbactam sodium antibiotic complex provided by the present invention and 100g piperacillin sodium / 400g sulbactam sodium respectively, compares grouping, 100 in each group, weighing 18-20g.
[0044] The raw materials that present embodiment adopts and the parts by weight of each raw material are as follows:
[0045] Piperacillin Sodium 100;
[0046] Sulbactam sodium 400;
[0047] Liposome 15;
[0048] Polyvinylpyrrolidone 15;
[0049] Reduced Glutathione 4.
[0050] The preparation method is as follows:
[0051] 1) Get the above-mentioned liposome solution containing 15g liposome.
[0052] 2) Add 100g of piperacillin sodium and 400g of sulbactam sodium into the liposome solution, and stir ultrasonically (the time of ultrasonic treatment should be less than 45 minutes, the treatment temperature should be...
Embodiment 2
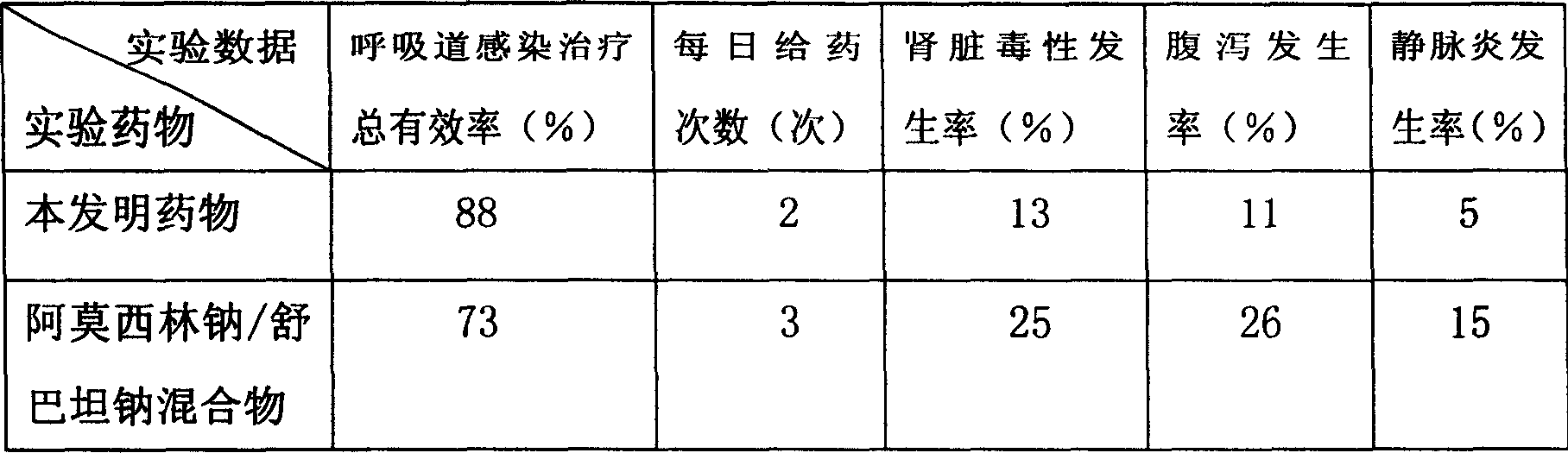
[0062] The present embodiment adopts the mouse of soft tissue infectious disease model as experimental animal, carries out comparative experiment with the sulbactam sodium antibiotic complex provided by the present invention and 400g amoxicillin sodium / 200g sulbactam sodium mixture respectively, compares grouping, each Group 100, body weight 18-20g.
[0063] The raw materials that present embodiment adopts and the parts by weight of each raw material are as follows:
[0064] Amoxicillin sodium 400;
[0066] Liposome 250;
[0067] Polyvinylpyrrolidone 250;
[0068] Reduced Glutathione 0.5.
[0069] The preparation method is as follows:
[0070] 1) Get the above-mentioned liposome solution containing 250g liposome.
[0071] 2) Add 400g of amoxicillin sodium and 200g of sulbactam sodium into the liposome solution, and stir ultrasonically (the time of ultrasonic treatment should be less than 45 minutes, the treatment temperature should be lower t...
Embodiment 3
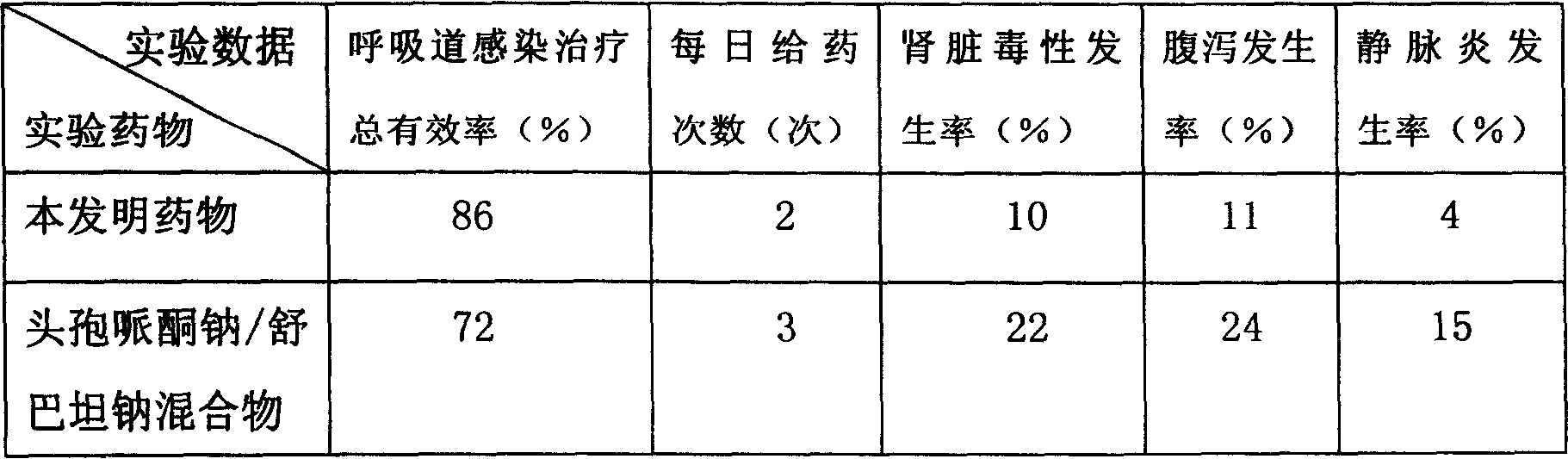
[0080] The present embodiment adopts the mouse of wound infection disease model as experimental animal, carries out comparative experiment with the sulbactam sodium antibiotic complex provided by the present invention and 200g cefoperazone sodium / 100g sulbactam sodium mixture respectively, compares grouping, every group 100 only, weighing 18-20g.
[0081] The raw materials that present embodiment adopts and the parts by weight of each raw material are as follows:
[0082] Cefoperazone Sodium 200;
[0083] Sulbactam Sodium 100;
[0084] Liposome 15;
[0085] Polyvinylpyrrolidone 15;
[0086] Reduced Glutathione 2.
[0087] The preparation method is as follows:
[0088] 1) Get the above-mentioned liposome solution containing 15g liposome.
[0089] 2) Add 200g of cefoperazone sodium and 100g of sulbactam sodium into the liposome solution, and dissolve under ultrasonic stirring (the time of ultrasonic treatment should be less than 45 minutes, the treatment temperature should...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com