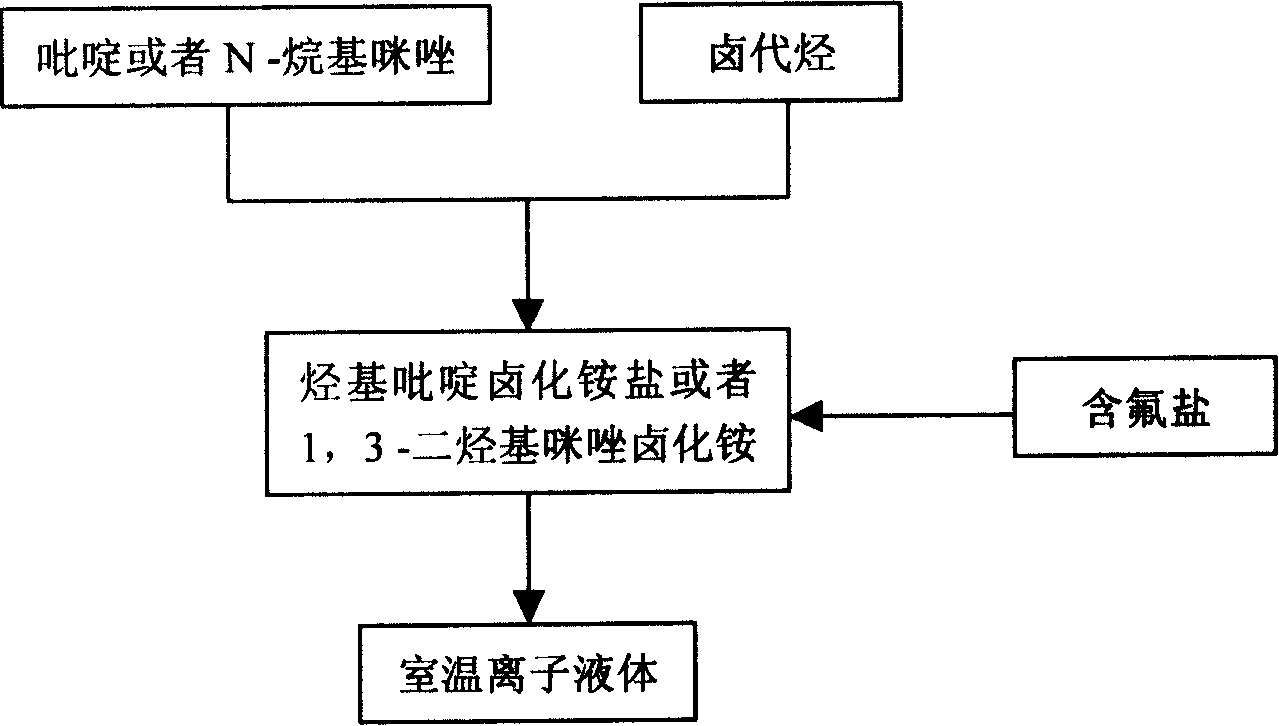
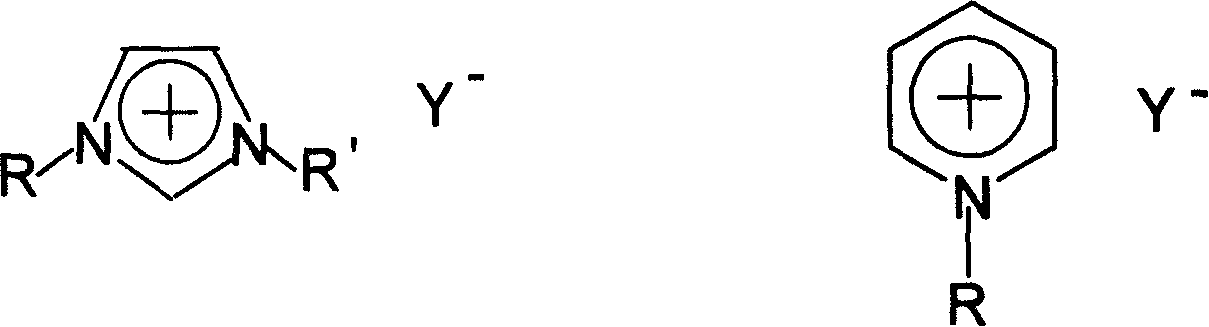Method of continuously synthesizing ionic liquid at room temperature
A technology of room temperature ionic liquid and synthesis method, applied in the field of continuous synthesis of room temperature ionic liquid, can solve the problems of difficult control of reaction conditions by microwave method, unspecified ionic liquid synthesis method, unfavorable industrialization promotion and application, etc., and is beneficial to large-scale industrialization. Production, elimination of separation and purification steps, and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 100 mL three-necked flask, add 13.7 g of n-bromobutane and 8.2 g of N-methylimidazole, and stir and react at 70-80° C. for 2 hours. Another 18.4 g of potassium hexafluorophosphate was added and stirring was continued for 2 hours. After the reaction was completed, 20 mL of water was added, and the water phase was left to stand to separate after fully stirring. The ionic liquid phase was washed three times with 20 mL×3 water respectively, and the residual water was removed under reduced pressure to obtain 26.4 g of alkylimidazole hexafluorophosphate ionic liquid. Yield 92.9%
Embodiment 2
[0023] In a 100 mL three-necked flask, 15.1 g of n-pentane bromide and 8.2 g of N-methylimidazole were added, and the reaction was stirred at 80° C. for 2 hours. Another 12.6 g of potassium tetrafluoroborate was added and stirring was continued for 2 hours. After the reaction was completed, 20 mL of acetone was added, stirred thoroughly, filtered, and the solvent was distilled off to obtain 21.6 g of alkylimidazole tetrafluoroborate ionic liquid with a yield of 90.2%.
Embodiment 3
[0025] In a 100 mL three-necked flask, 13.7 g of n-bromobutane and 8.2 g of N-methylimidazole were added, and the mixture was stirred and reacted at 110° C. for 2 hours. Another 11.0 g of sodium tetrafluoroborate was added and stirring was continued for 2 hours. After the reaction was completed, 20 mL of acetone was added, stirred thoroughly, filtered, and the solvent was removed by distillation to obtain 21.5 g of alkylpyridine tetrafluoroborate ionic liquid with a yield of 85.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com