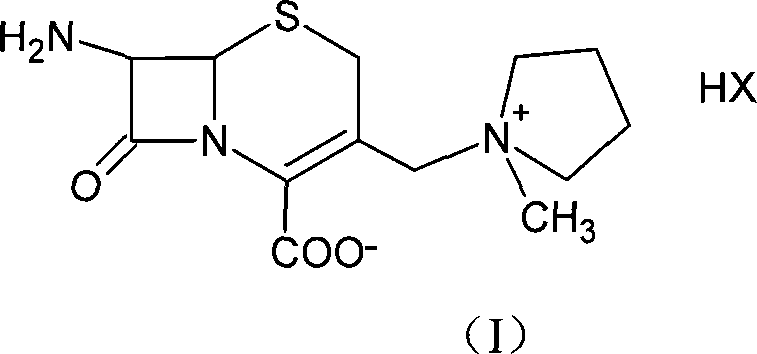
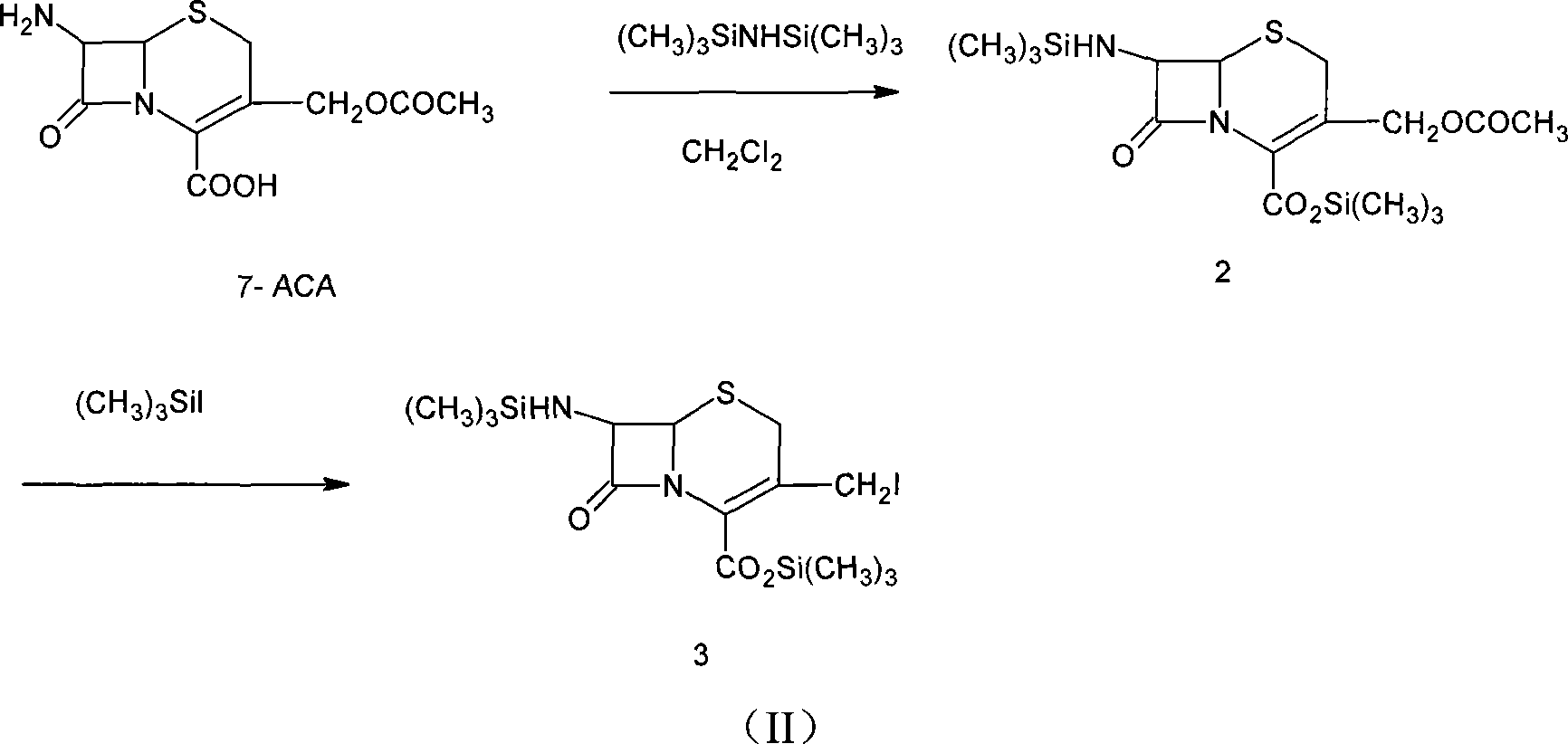
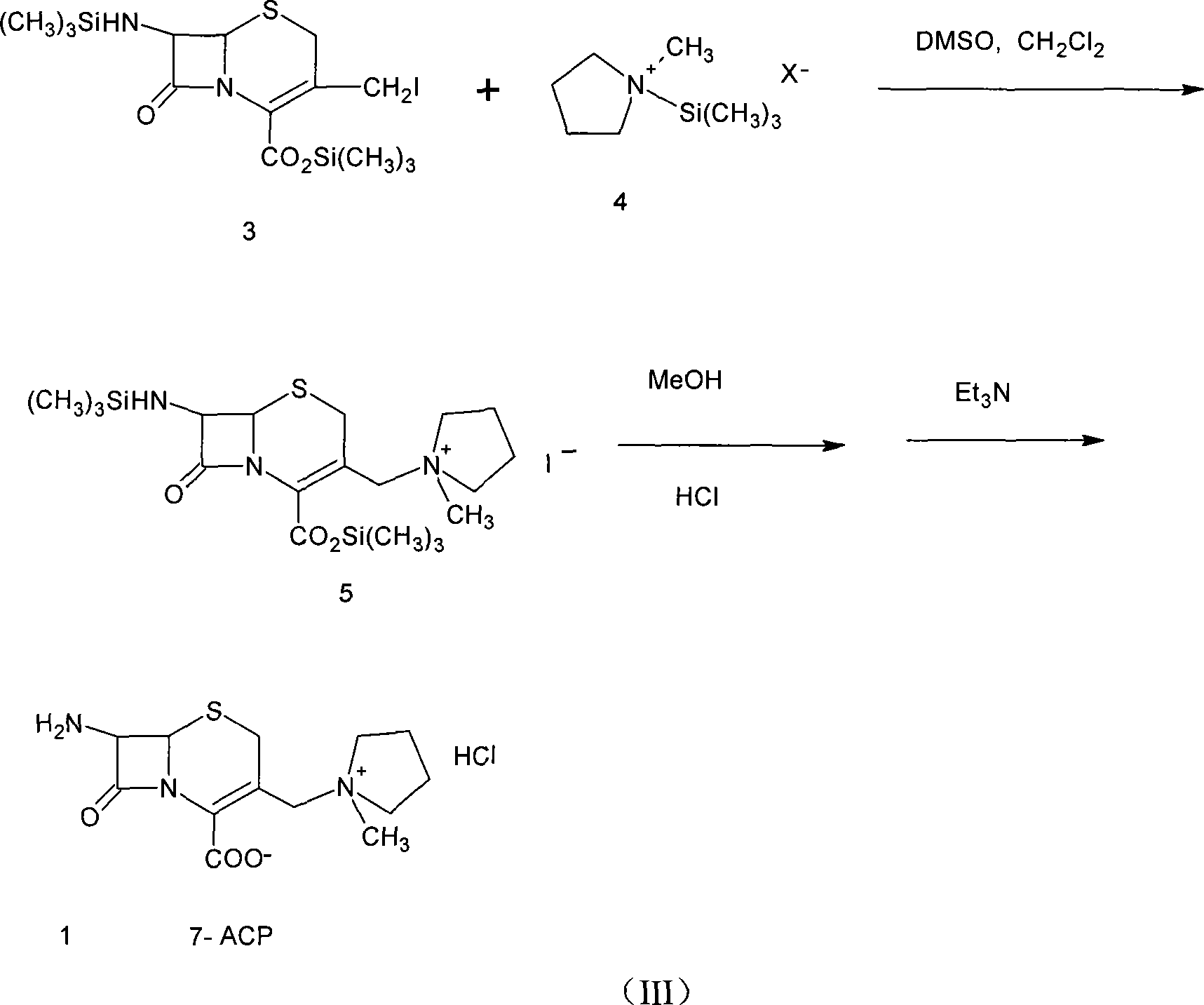
Method of synthesizing cefepime intermediate in mixed solvent
A synthesis method and mixed solvent technology, which is applied in the synthesis of intermediates and the field of synthesis of -7-amino-3-[methyl]ceph-3-ene-4-carboxylate hydrochloride, can solve the problem of long reaction time , Affect product quality and yield, product color and poor quality and other issues, to achieve high quality, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: (6R, 7R)-7-amino-3-[(1-methyl-1-pyrrolidine)methyl]ceph-3-ene-4-carboxylate hydrochloride (1)(7- ACP) preparation
[0031]In a 500mL four-neck flask equipped with a stirrer, condenser, thermometer and dropping funnel, add 40g (0.147mol) of 7-ACA and 200mL of dichloromethane, and add 36mL (0.170mol) of hexamethyldisilamine at room temperature 20°C Alkanes (HMDS) and 0.2mL trimethyl iodosilane (TMSI), heated to reflux for 8-12hrs under the protection of nitrogen, after checking that no ammonia gas is released, cool the solution to 0-5°C, and add it slowly under the protection of nitrogen 26.3 mL (0.183 mol) of iodotrimethylsilane (TMSI), kept at a temperature not exceeding 10-25°C, and reacted for 2 hours while stirring and keeping warm. HPLC detection 7-ACA iodide has been completed. Dichloromethane was recovered under reduced pressure, 130-150 mL was distilled, and the distillation temperature was lower than 20°C. The reaction solution was cooled to -30°C,...
Embodiment 2
[0034] Example 2: (6R, 7R)-7-amino-3-[(1-methyl-1-pyrrolidine) methyl] ceph-3-ene-4-carboxylic acid iodate (7-ACP) preparation
[0035] In a 500mL four-neck flask equipped with a stirrer, condenser, thermometer and dropping funnel, add 40g (0.147mol) of 7-ACA and 200mL of dichloromethane, and add 36mL (0.170mol) of hexamethyldisilamine at room temperature 20°C Alkanes (HMDS) and 0.2mL trimethyl iodosilane (TMSI), heated to reflux for 8-12hrs under the protection of nitrogen, after detecting that no ammonia gas is released, cool the solution to 0-5°C, and under the protection of nitrogen, slowly add 26.3 mL (0.183 mol) of iodotrimethylsilane (TMSI), kept at a temperature not exceeding 10-25°C, and reacted for 2 hours while stirring and keeping warm. HPLC detection 7-ACA iodide has been completed. Dichloromethane was recovered under reduced pressure, and 130-150 mL was distilled out, and the distillation temperature was lower than 20°C. The reaction solution was cooled to -30...
Embodiment 3
[0037] Example 3: (6R, 7R)-7-amino-3-[(1-methyl-1-pyrrolidine)methyl]ceph-3-ene-4-carboxylate hydrochloride (1)(7- ACP) preparation
[0038] In a 500mL four-neck flask equipped with a stirrer, condenser, thermometer and dropping funnel, add 40g (0.147mol) of 7-ACA and 200mL of dichloromethane, and add 36mL (0.170mol) of hexamethyldisilazol at room temperature 20°C Amane (HMDS) and 0.2mL trimethyl iodosilane (TMSI), heated to reflux for 8-12hr under the protection of nitrogen, after detecting that no ammonia gas is released, cool the solution to 0-5°C, under the protection of nitrogen, stir slowly Add 26.3 mL (0.183 mol) of iodotrimethylsilane (TMSI), keep the temperature not exceeding 10-25° C., and react for 2 h under stirring and heat preservation. HPLC detection 7-ACA iodide has been completed. Dichloromethane was recovered under reduced pressure, 130-150 mL was distilled, and the distillation temperature was lower than 20°C. The reaction solution was cooled to -30°C, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com