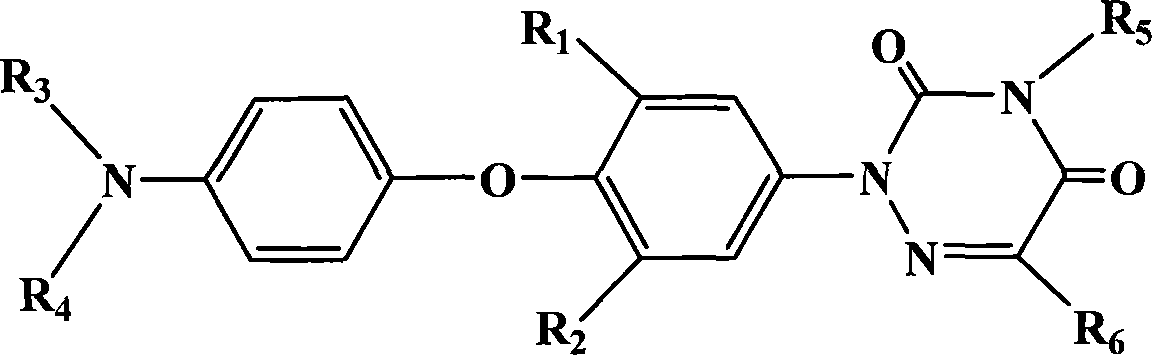
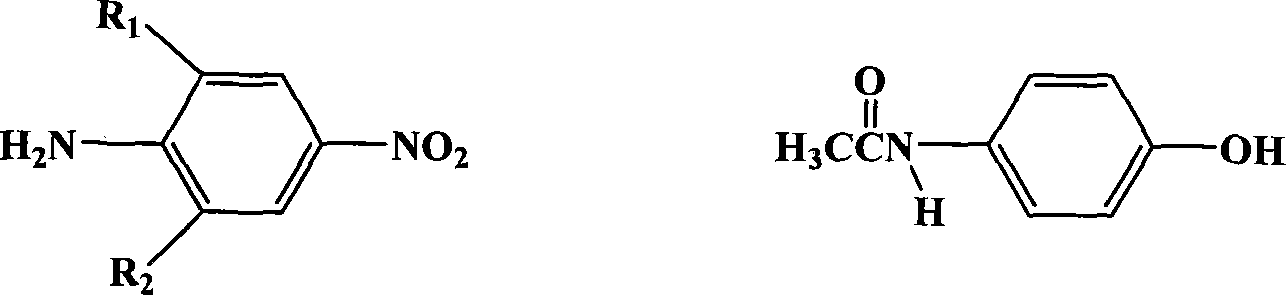Compound in triazine class for anti activity of coccidian, preparation method and application
A compound, anti-coccidial technology, applied in the direction of active ingredients of heterocyclic compounds, organic chemistry, anti-infective drugs, etc., can solve the problems of shortening the service life of anti-coccidial drugs, affecting exports, and failure of prevention and control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Synthesis of 2-[4-(4′-dichloroacetamidophenoxy)-3-methyl-phenyl]-1,2,4-triazine-3,5-dione
[0069] Step A: Synthesis of 2-methyl-4-nitro-4'-acetamido-diphenyl ether
[0070] Dissolve 12.6 g of potassium oxide particles in 250 ml of DMSO, and heat to 80° C. with stirring. 24g of acetaminophen was dissolved in 40ml of DMSO, and slowly added to the reaction solution. After the addition, the temperature was raised to reflux for 30 minutes, and 150 benzene was added to divide the water. 27.5 g of 2-chloro-5-nitrotoluene was dissolved in 80 ml of DMSO, then added dropwise to the above reaction solution, and reacted at room temperature for 10 hours. Afterwards, the reaction solution was poured into 2.5 L of water, stirred vigorously for 1 hour, the crude product was filtered out, washed with water until the filtrate was colorless, and dried. The crude product was recrystallized from ethanol / water to obtain 38.9 g of white solid with a yield of 86%. Melting point: 165.5-166...
Embodiment 2
[0082] Synthesis of 2-[4-(4′-dichloroacetamidophenoxy)-3,5-dichloro-phenyl]-1,2,4-triazine-3,5-dione
[0083] The operation steps are the same as in Example 1, and in step A, 3,4,5-trichloro-nitrobenzene is used as a reactant.
[0084] Melting point: 226.0~227.8℃;
[0085] ES-MS (m / z): 473.4 (M-H) - .
Embodiment 3
[0087] Synthesis of 2-[4-(4′-thiazolaminophenoxy)-3-methyl-5-chloro-phenyl]-1,2,4-triazine-3,5-dione
[0088] The operation steps are the same as in Example 1, and in step A, 2,3-dichloro-5-nitrotoluene is used as the reactant.
[0089] Step F: Dissolve 4.54 g of 2-[4-(4'-aminophenoxy)-3-methyl-5-chloro-phenyl]-1,2,4-triazine-3,5-dione Add 1ml of pyridine to 20ml of anhydrous DMF, add 2.3g of 2-bromothiazole dropwise at room temperature, drop it in 30 minutes, rise to 80°C for 4 hours, add 30ml of water, filter after cooling, dry, methanol / Recrystallized from water to obtain 2.6 g of a light yellow product with a yield of 62%.
[0090] Melting point: 252.3~254.5℃;
[0091] ES-MS (m / z): 427.4 (M-H) - .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com