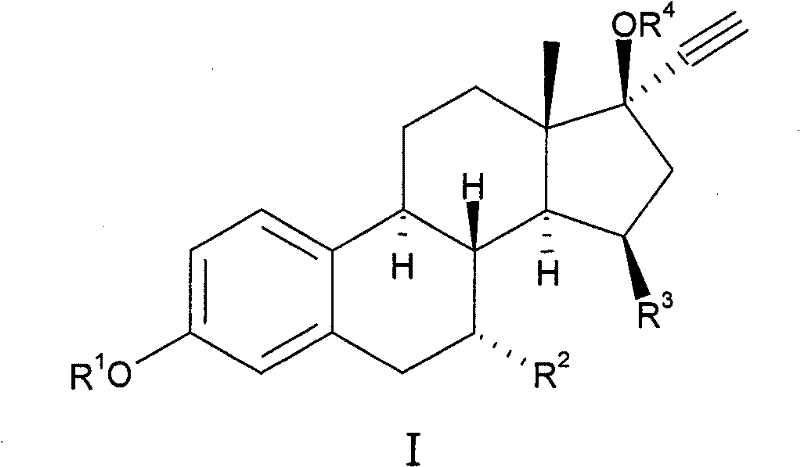
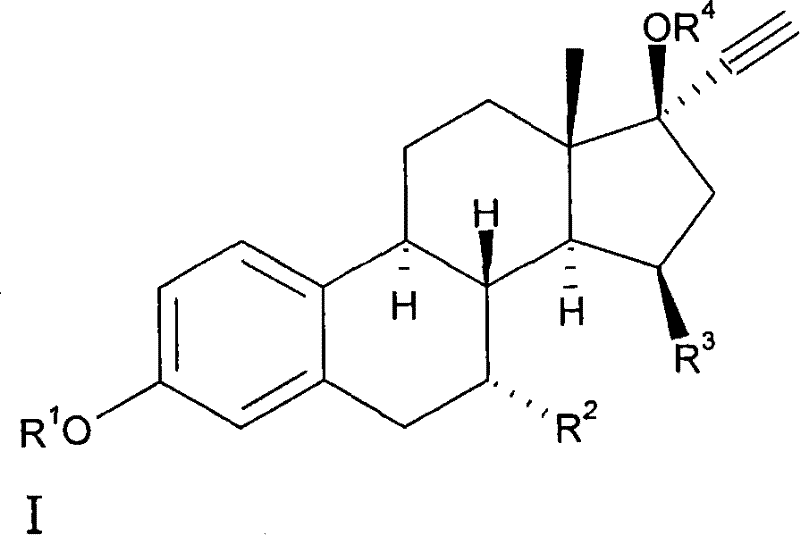
15beta-substituted steroids having selective estrogenic activity
A technology of steroid and hormone therapy, applied in the direction of organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., can solve problems such as increasing the risk of breast cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
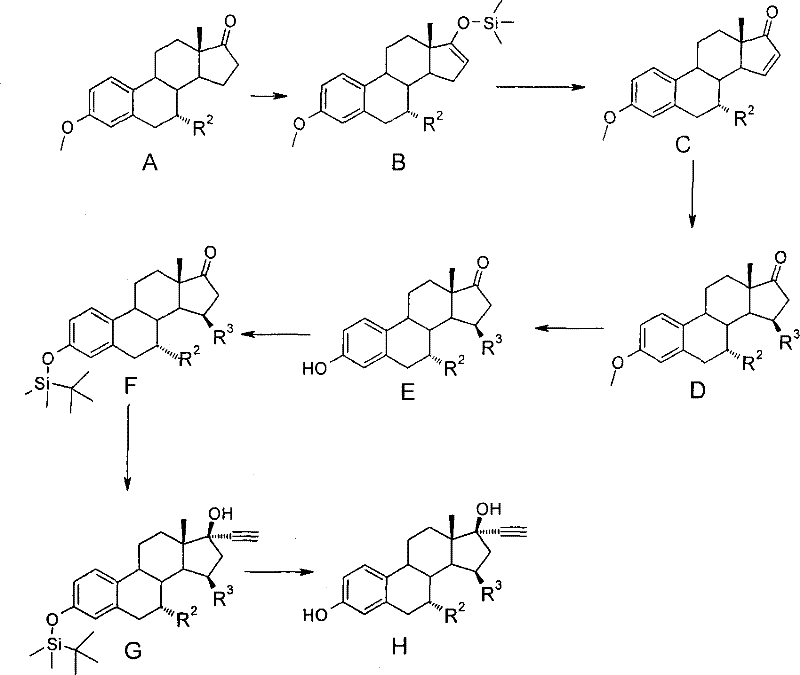
[0061] Preparation of 7α-ethyl-15β-methyl-19-nor-17α-pregna-1,3,5(10)-triene-20-yne-3,17β-diol (8) (see Scheme II ).
[0062] Preparation of 7α-ethyl-3-methoxy-estr-1,3,5(10),15-tetraen-17-one (3).
[0063] From 17β-17-(acetyloxy)-estra-4,6-dien-3-one and ethyl bromide in a manner similar to that described in EP 0869132A1 (see Example I and Scheme I, compounds 1-5) Preparation of 7α-ethyl-3-methoxyestrone 1 from base magnesium.
[0064] To a solution of LDA [prepared by adding a 1.6 M solution of n-butyllithium in heptane (4.7 ml) to diisopropylamine (2.1 ml) in THF (15 ml) at -50 °C] dropwise A solution of 7a-ethyl-3-methoxyestrone 1 (1 g) in THF (3 ml) was added. The mixture was stirred at -60°C for half an hour and then treated with trimethylsilyl chloride (2ml). The reaction mixture was warmed to 0 °C within half an hour, then poured into 10% NH 4 Cl aqueous solution (100ml), extracted with ethyl acetate. Washed, dried (Na 2 SO 4 ), followed by concentration to pro...
Embodiment 2
[0075] Preparation of 3-pivaloyloxy-7α-ethyl-15β-methyl-19-nor-17α-pregna-1,3,5(10)-triene-20-yne 17β-ol (9a)
[0076] Compound 8 (300mg) was dissolved in pyridine (10ml). Pivaloyl chloride (1.5 equiv) was added dropwise. After 2 hours, the reaction mixture was quenched with water. The reaction mixture was concentrated, redissolved in ethyl acetate, and extracted with aqueous sodium bicarbonate and water. dry (Na 2 SO 4 ) and concentrate the organic layer. Purification of the residue by chromatography on silica gel (heptane-ethyl acetate (1:0 -> 4:1) yielded pure 9a (347 mg). NMR (CDCl 3 ) δ 1.35 (s, 9H, pivaloyl), 1.08 (d, 3H, 15β-Me), 1.02 (s, 3H, 18-Me), 0.94 (t, 3H, 7-ethyl).
[0077] Compound 9b (289 mg; NMR (CDCl 3 ) δ3.0 and 3.08 (2xs, 6H, NMe 2 ) 1.08 (d, 3H, 15β-Me), 1.02 (s, 3H, 18-Me), 0.93 (t, 3H, 7-ethyl)) and 9c (283 mg; NMR (CDCl 3 )δ4.32(q, 2H, OCH 2 CH 3 ), 1.38 (d, 3H, OCH 2 CH 3 ), 1.08 (d, 3H, 15β-Me), 1.02 (s, 3H, 18-Me), 0.93 (t, 3H, 7-ethyl)...
Embodiment 3
[0079] The agonistic activity of the compounds on the estrogen receptor was determined in an in vitro bioassay using recombinant Chinese hamster ovary (CHO) cells prepared with human estrogen receptor α-(hERα-) or β-(hERβ-) , rat oxytocin-promoting factor (RO) and luciferase reporter gene (LUC) stably co-transfected cells. The potency of the test compound to stimulate luciferase through estrogen receptor hERα- or hERβ-mediated transactivation, i.e. estrogen agonistic transactivation is expressed as EC relative to the standard estrogen 17β-estradiol 50 Percentage (%) of (potency of test compound=(EC of 17β-estradiol 50 / EC of test compound 50 )×100%). Efficacy, i.e. the amount of maximal activation of the receptor induced by the compound, is expressed as a percentage (%) relative to the maximal activation induced by the standard estrogen 17β-estradiol (efficacy of test compound = (test Maximum activation of compound / maximum activation of 17β-estradiol)×100%). A more thorou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com