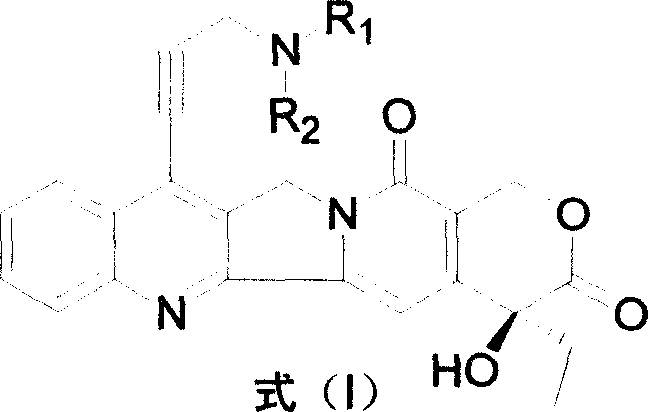
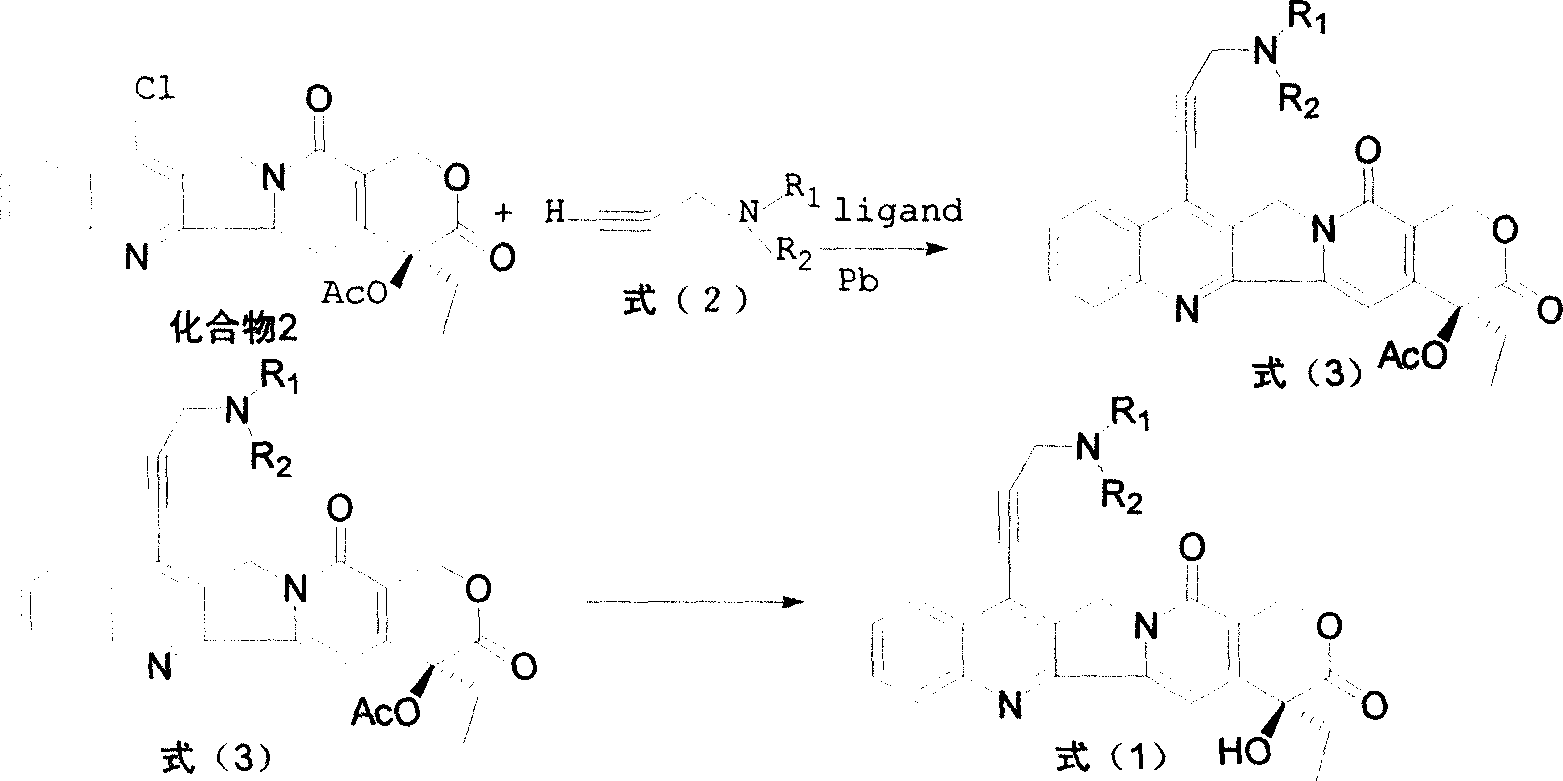
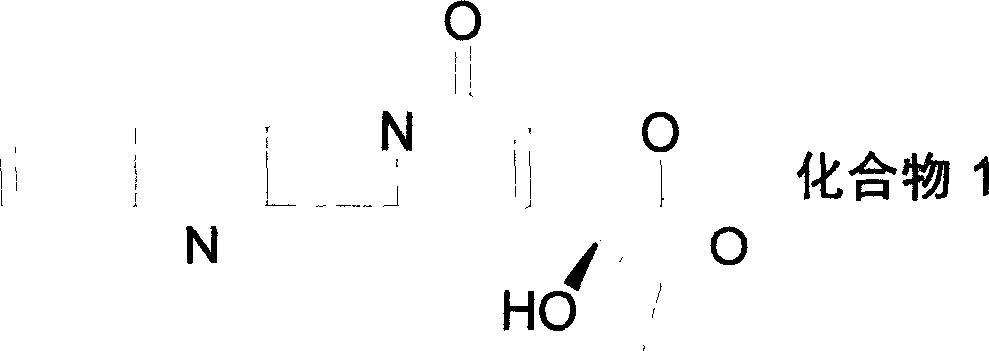
Water-soluble camptothecine derivative and its preparation process and application thereof
A camptothecin, water-soluble technology, applied in the direction of drug combination, pharmaceutical formula, organic active ingredients, etc., can solve the problem of inability to prepare camptothecin derivatives, etc., and achieve low cost, good water solubility, and broad anti-tumor spectrum Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: the preparation of 7-(3'-dimethylaminopropynyl) camptothecin (CPT-11)
[0052] Add 500mg of 7-chloro-20-acetylcamptothecin, 40mg of palladium acetate, 220mg of BINAP, and 325mg of anhydrous potassium carbonate into the flask, under nitrogen protection, add 0.40ml of 3-dimethylaminopropyne, 75mL of toluene, reflux reaction 22 Hours, the detection reaction was complete, cooled, filtered, and the toluene was evaporated under reduced pressure, and the residue was subjected to silica gel column chromatography, eluted with chloroform:methanol (200:1) to obtain 7-(3'-dimethylaminopropynyl)- 20-acetyl camptothecin 400mg.
[0053]The above product was added to 20 mL of concentrated sulfuric acid, stirred at room temperature until the reaction was complete, the reaction mixture was added to crushed ice, neutralized with NaOH aqueous solution, extracted with chloroform, and column chromatographed to obtain 250 mg of 7-(3'-dimethyl Aminopropynyl) camptothecin (CPT-11...
Embodiment 2
[0056] Embodiment 2: 7-(3'-piperidinyl propynyl) camptothecin (CPT-21) preparation:
[0057] Add 500mg of 7-chloro-20-acetylcamptothecin, 40mg of palladium acetate, 220mg of BINAP, and 325mg of anhydrous potassium carbonate into the flask, under nitrogen protection, add 0.45ml of 3-piperidylpropyne, 75mL of toluene, and reflux reaction 22 After 1 hour, the detection reaction was complete, cooled, filtered, and the toluene was evaporated under reduced pressure, and the residue was subjected to silica gel column chromatography, eluted with chloroform:methanol (200:1) to obtain 7-(3'-piperidinylpropynyl)-20 - Acetyl camptothecin 460mg.
[0058] The above product was added to 20 mL of concentrated sulfuric acid, stirred at room temperature until the reaction was complete, the reaction mixture was added to crushed ice, adjusted to neutrality with NaOH aqueous solution, extracted with chloroform, and column chromatographed to obtain 300 mg of 7-(3'-piperidine ylpropynyl) camptothec...
Embodiment 3
[0061] Example 3: 7-(3'-diisopropylaminopropynyl)camptothecin (CPT-17)
[0062] Add 500mg of 7-chloro-20-acetylcamptothecin, 40mg of palladium acetate, 220mg of BINAP, and 325mg of anhydrous potassium carbonate into the flask, under nitrogen protection, add 0.50ml of 3-diisopropylaminopropyne, 75mL of toluene, and reflux reaction After 22 hours, the reaction was detected to be complete, cooled, filtered, and the toluene was evaporated under reduced pressure, and the residue was subjected to silica gel column chromatography, eluted with chloroform:methanol (200:1) to obtain 7-(3'-diisopropylaminopropyne base)-20-acetylcamptothecin 300mg.
[0063] The above product was added to 20 mL of concentrated sulfuric acid, stirred at room temperature until the reaction was complete, the reaction mixture was added to crushed ice, neutralized with NaOH aqueous solution, extracted with chloroform, and column chromatographed to obtain 150 mg of 7-(3'-diiso Propylaminopropynyl) camptothecin ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com