Process for producing phenol and methyl ethyl ketone
一种甲乙酮、苯酚的技术,应用在共同制备苯酚和甲乙酮领域,能够解决没有异丁基苯或叔丁基苯杂质含量的公开内容等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
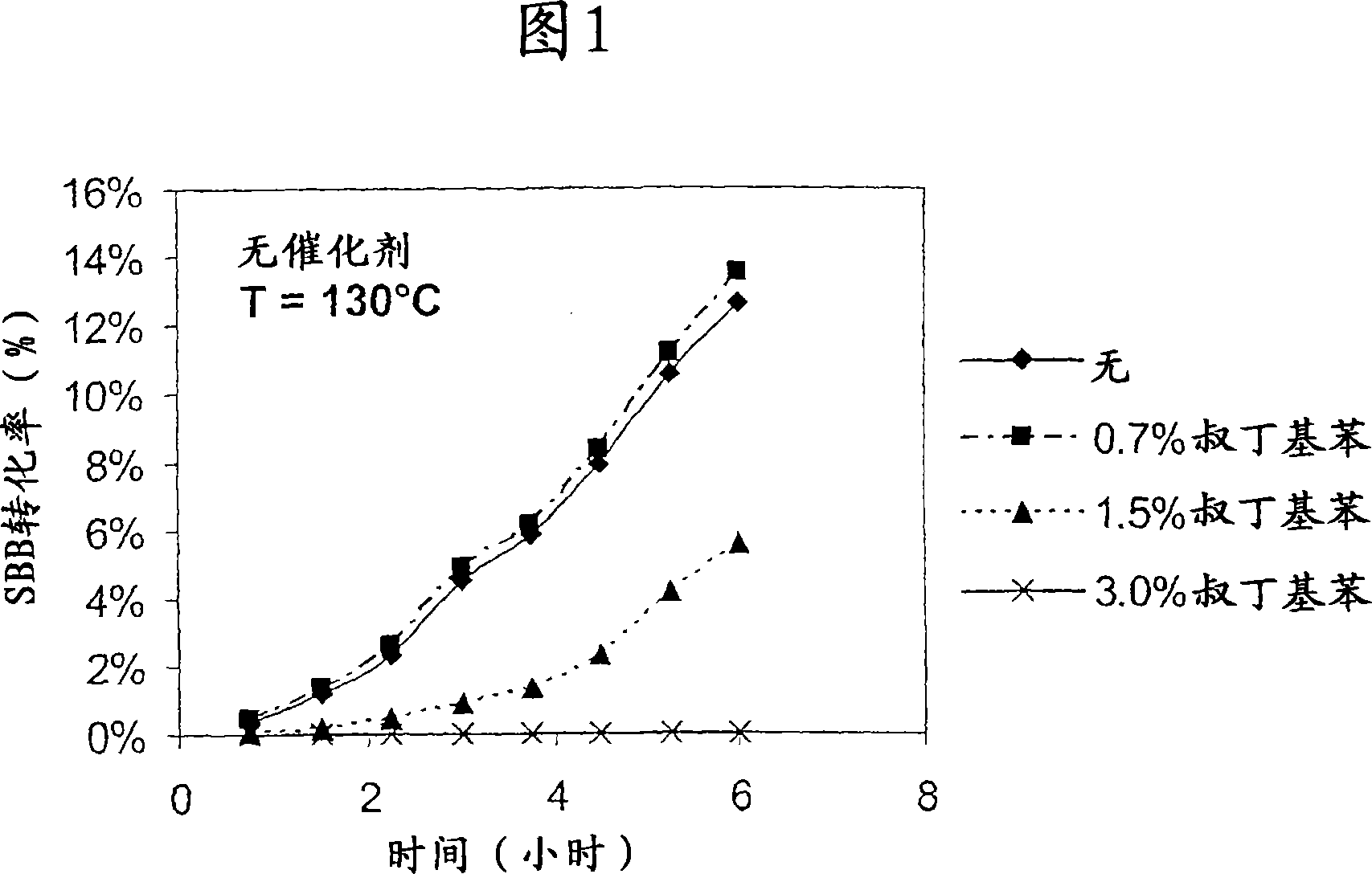
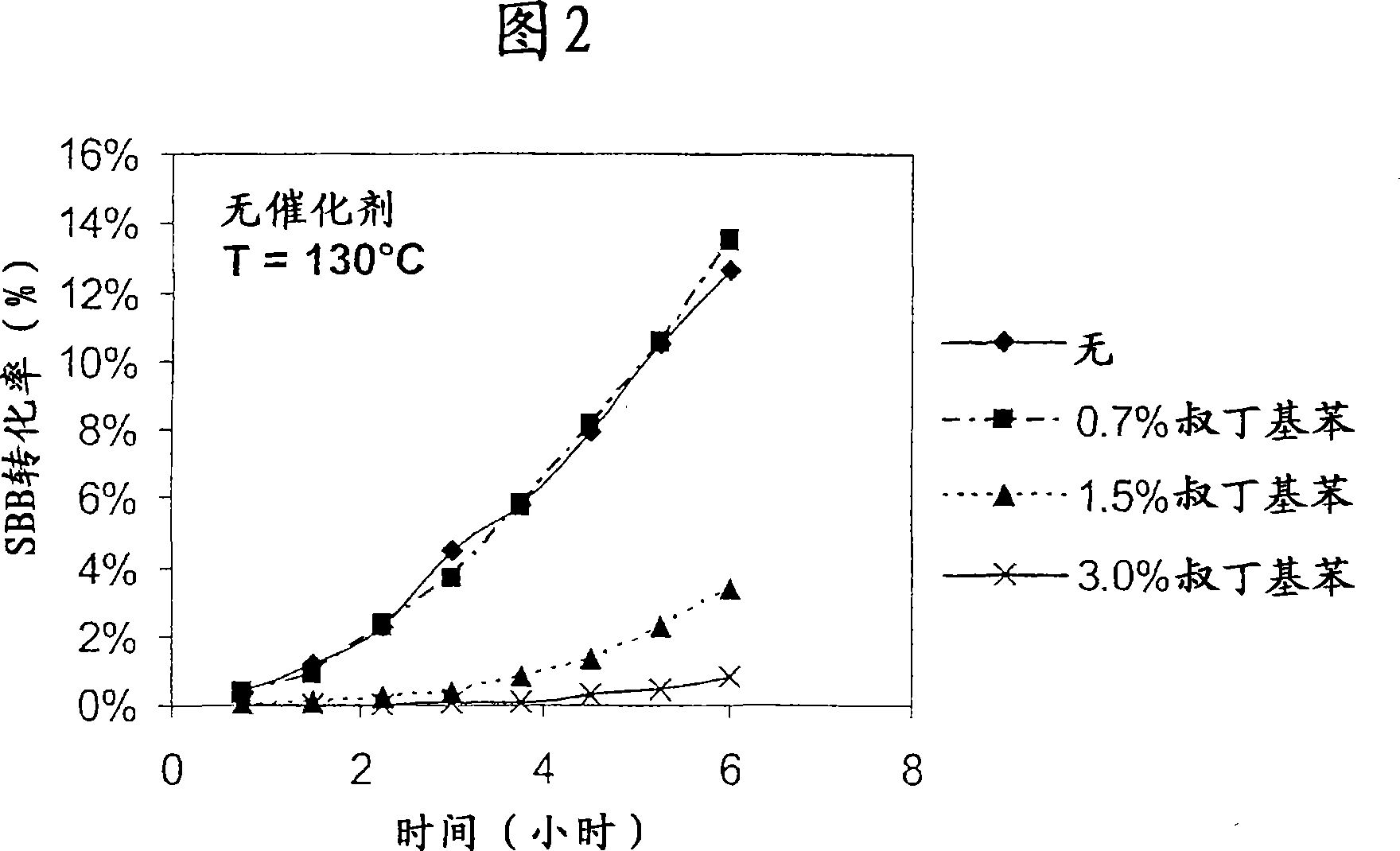
Image
Examples
Embodiment 1
[0088] Example 1: Synthesis of sec-butylbenzene using MCM-22
[0089] A 0.5 g sample of MCM-22 catalyst (65 wt% MCM-22 / 35% alumina binder) was used to alkylate benzene with 2-butene. The catalyst was in the form of a 1.6 mm (1 / 16") diameter cylindrical extrudate and was diluted to 3 cc with sand and loaded into an isothermal, down-flow, fixed bed with an outer diameter of 4.76 mm (3 / 16") in the tubular reactor. The catalyst was dried with 100 cc / min flowing nitrogen at 125°C and 1 atm for 2 hours. The nitrogen was turned off and benzene was fed to the reactor at 60 cc / hr for 1 hour then reduced to the desired WHSV while increasing the reactor pressure to 300 psig (2170 kPa). 2-Butene (a mixture of cis and trans forms) was introduced from a syringe pump at a 3:1 benzene / butene molar ratio and the reactor temperature was ramped at 5°C / min to 160°C. The liquid product was collected in a cold trap and analyzed off-line. Butene conversion was determined by measuring unreacted b...
Embodiment 2
[0090] Example 2: Synthesis of sec-butylbenzene using zeolite beta
[0091] The procedure of Example 1 was repeated, but the MCM-22 catalyst was replaced by 0.5 g zeolite beta catalyst (65 wt% beta / 35% alumina binder), which was also extruded in a 1.6 mm (1 / 16") diameter cylinder Catalyst performance on days 1, 3 and 5 in production is shown in Table 2.
[0092] Table 2
[0093] catalyst
MCM-22
Zeolite beta
days in production
Butene WHSV, h -1
2-butene conversion, %
10
1.5
95.8
13
1.5
96.4
1
2.0
97.5
3
2.0
70.4
5
2.0
48.5
Product selectivity, wt%
Isobutene & 1-Butene
C 5 -C 7
C 8 and C 12 (butene oligomer)
tert-butylbenzene
Isobutylbenzene*
sec-butylbenzene
n-Butylbenzene
Dibutylbenzene
tributylbenzene
other
total
0.049
0.077
2.199
0.069
0.099
0.000...
Embodiment 3
[0096] Example 3: Synthesis of sec-butylbenzene using MCM-22
[0097] A 1.0 g sample of the same MCM-22 catalyst (65 wt% MCM-22 / 35% alumina binder) as used in Example 1 was used to alkylate benzene with 2-butene. The catalyst was in the form of a 1.6mm (1 / 16") diameter cylindrical extrudate, cut to 1 / 16" in length, and diluted to 3cc with sand and filled with an outer diameter of 4.76m (3 / 16") In the isothermal, down-flow, fixed-bed tubular reactor. Dry the catalyst with 100cc / min flowing nitrogen at 150°C and 1 atm for 2 hours. Close the nitrogen and send benzene into the reactor at 60cc / hr for 1 hour and then Decrease to desired WHSV while increasing reactor pressure to 300 psig (2170 kPa). Butene feed (57.1% cis-butene, 37.8% trans -butene, 2.5% n-butane, 0.8% isobutene and 1-butene, and 1.8% other substances), and this ratio remained constant throughout the test. The reactor temperature was ramped up to 160 at 2°C / min C. Liquid product was collected in a cold trap and an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| degree of oxidation | aaaaa | aaaaa |
| degree of oxidation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com