Recombinant human keratinized cell growth factor production method
A technology for keratinocytes and growth factors, applied in the field of bioactive polypeptides of human keratinocyte growth factors prepared by gene recombination technology, can solve the problems of toxicity, complicated process steps, low KGF expression efficiency, etc., to simplify the production process and improve production efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
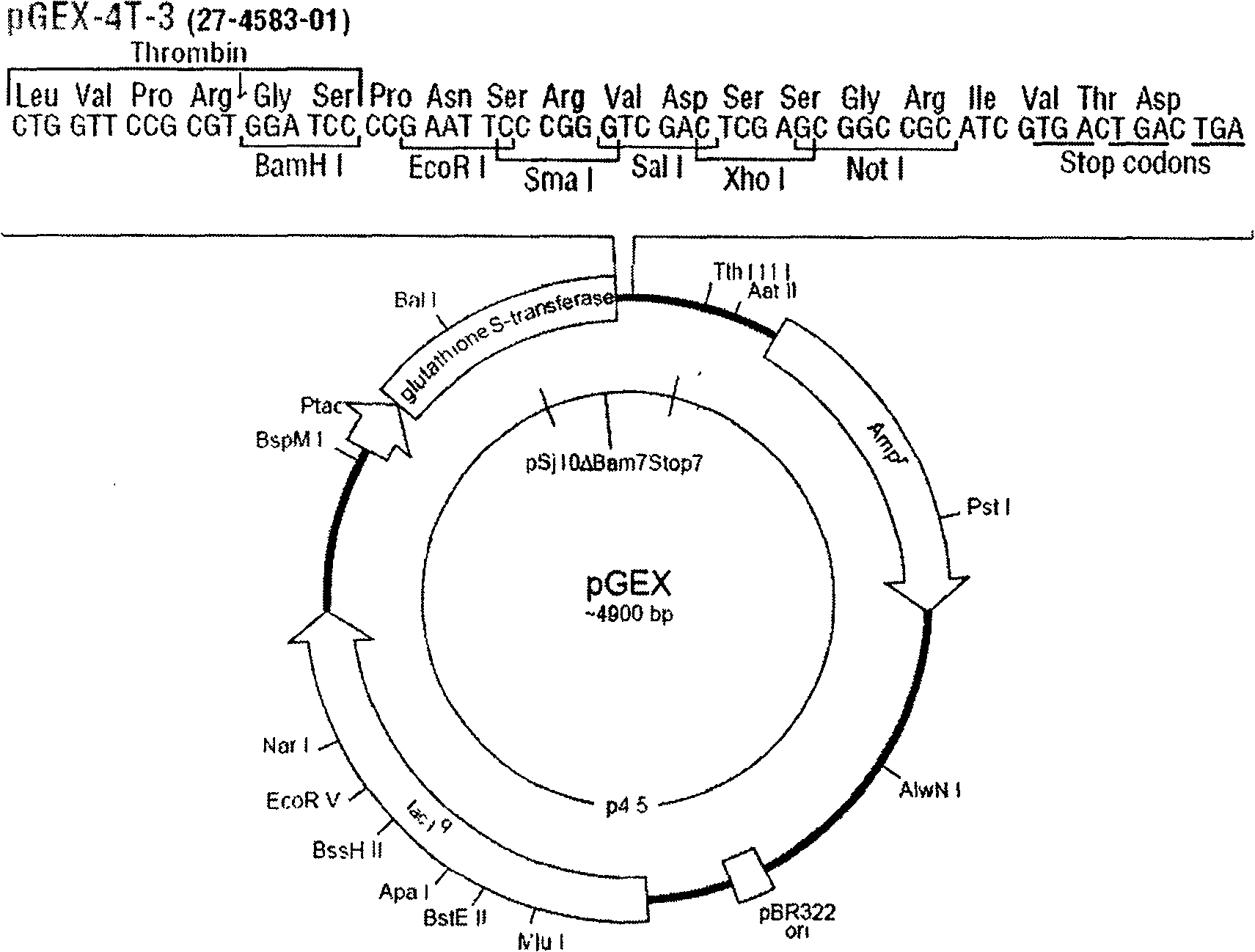
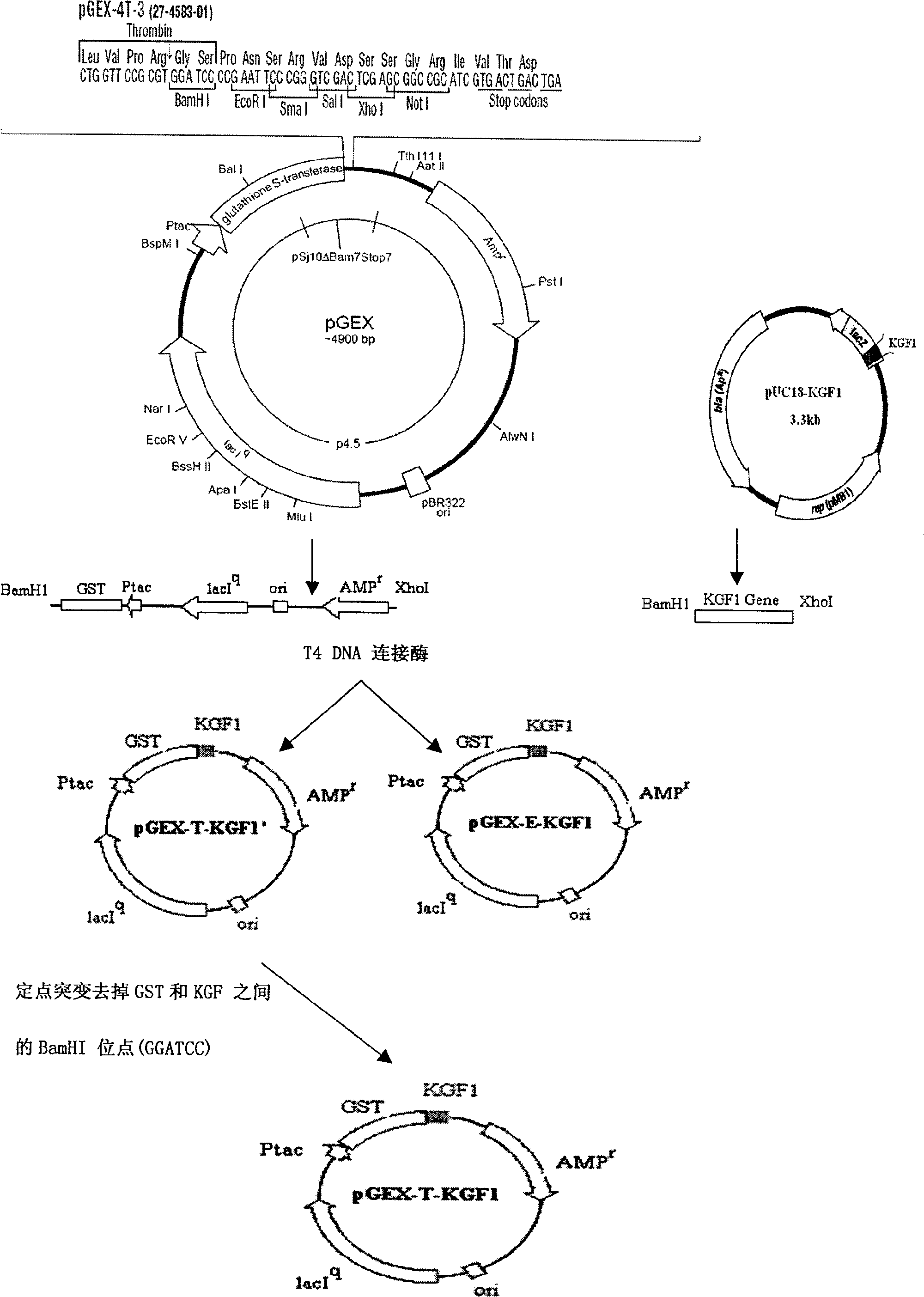
[0042] 1. Selection of expression vector
[0043] Choose Novagen’s pGEX series expression vectors such as pGEX-4T-3 as the expression vector. pGEX-4T-3 is an E. coli expression vector system for efficient fusion expression of proteins and polypeptides. The target protein or polypeptide is 225 amino acid residues Glutathione S-transferase (GST) was used as the fusion partner. (see attached figure 1 ).
[0044] 2. Gene design and synthesis:
[0045]According to the KGF1 DNA coding sequence reported in the literature (the sequence can be changed according to the principle of universality and degeneracy of the genetic code, but the amino acid composition and arrangement are not changed), the code is designed to mature into the KGF1 sequence, and BamHI is added to the 5-end of the sequence Recognition sequence GGATCC and enterokinase (Enterokinase) cleavage site coding sequence or thrombin (thrombin) cleavage site coding sequence; add the recognition sequence CTCGAG of Xho I at ...
Embodiment 2
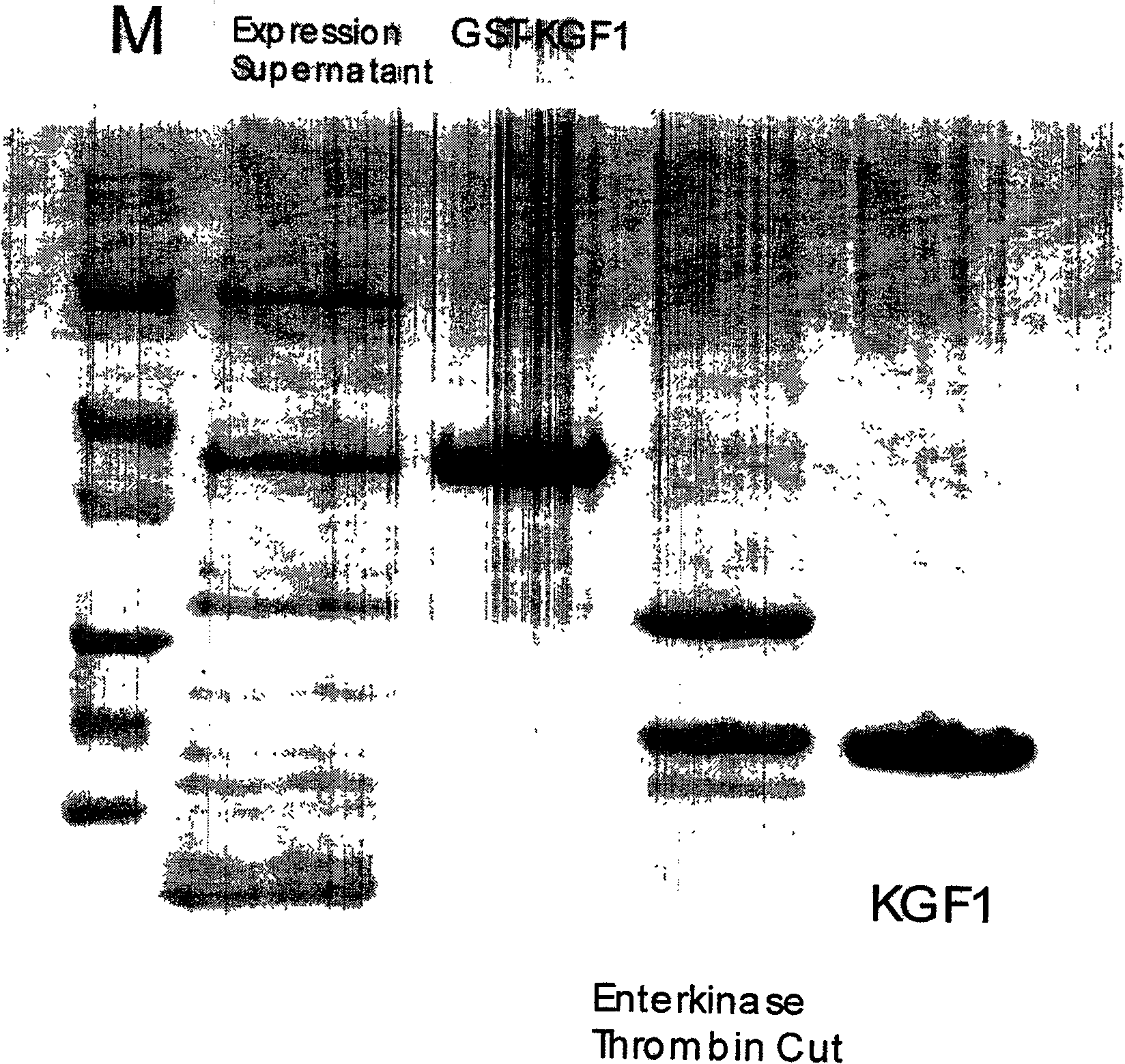
[0051] According to the physicochemical property determination of the rhKGF of embodiment 1 gained
[0052] 1. Determination of the N-terminal 15 amino acid sequences
[0053] The recombinant human keratinocyte growth factor obtained by the above two methods was subjected to N-terminal assay, and the assay results were as follows:
[0054] N-Ser-Tyr-Asp-Tyr-Met-Glu-Gly-Gly-Asp-Ile-Arg-Val-Arg-Arg-Leu-C
[0055] exactly as expected.
[0056] 2. Quantitative mass spectrometry
[0057] The molecular weight of the recombinant human keratinocyte growth factor obtained by the two methods was determined by FAB method, which was 16278.5, which was consistent with the theoretical prediction.
[0058] 3. Determination of biological activity:
[0059] The biological activity of KGF1 was determined by using the characteristics of KGF1 to promote the proliferation of 4MBr-5 cells.
[0060] The measured results are:
[0061]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com