Adefovir dipivoxil CHARIOTEER crystallographic form and preparation method thereof
A technology of adefovir dipivoxil and its crystal form, which is applied in chemical instruments and methods, organic chemistry, and compounds of elements of group 5/15 of the periodic table, etc., can solve the problem of high cost of preparation of adefovir dipivoxil crystal form, lack of Suitable for large-scale industrial production, unfavorable environmental protection and other issues, to achieve good crystal form stability, no reduction in content, and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
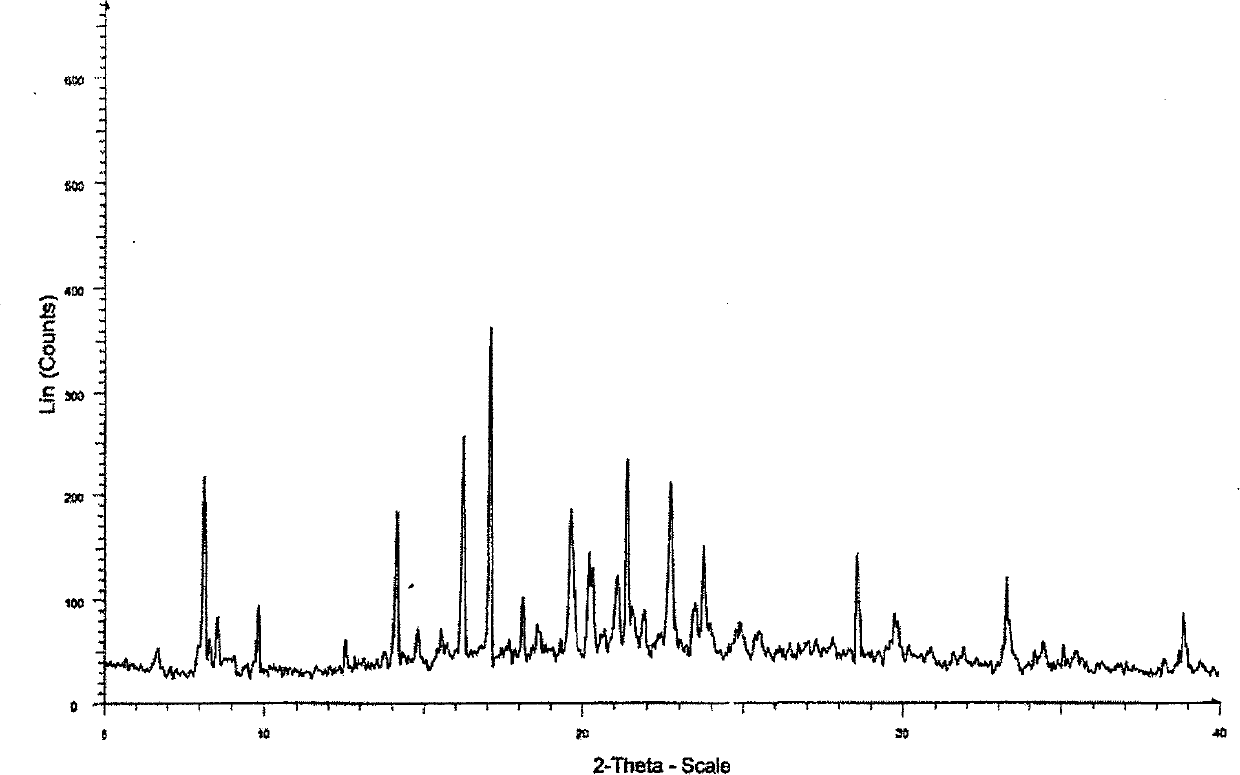
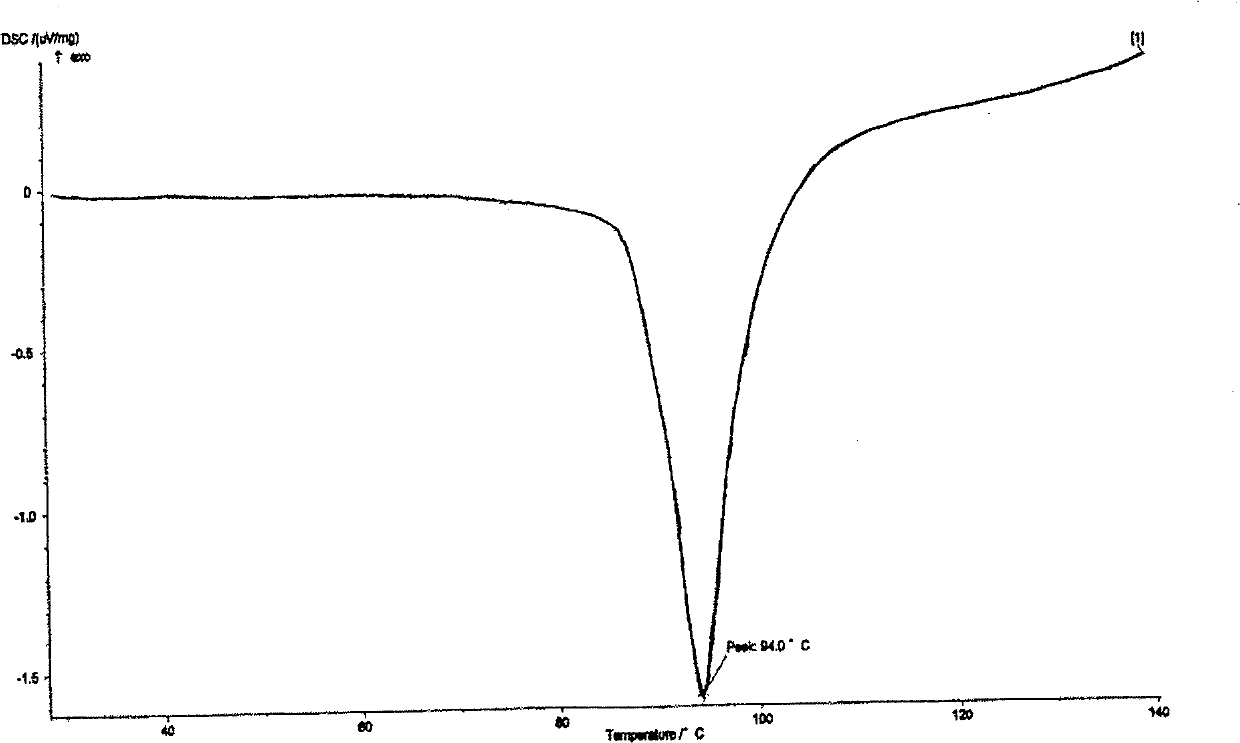
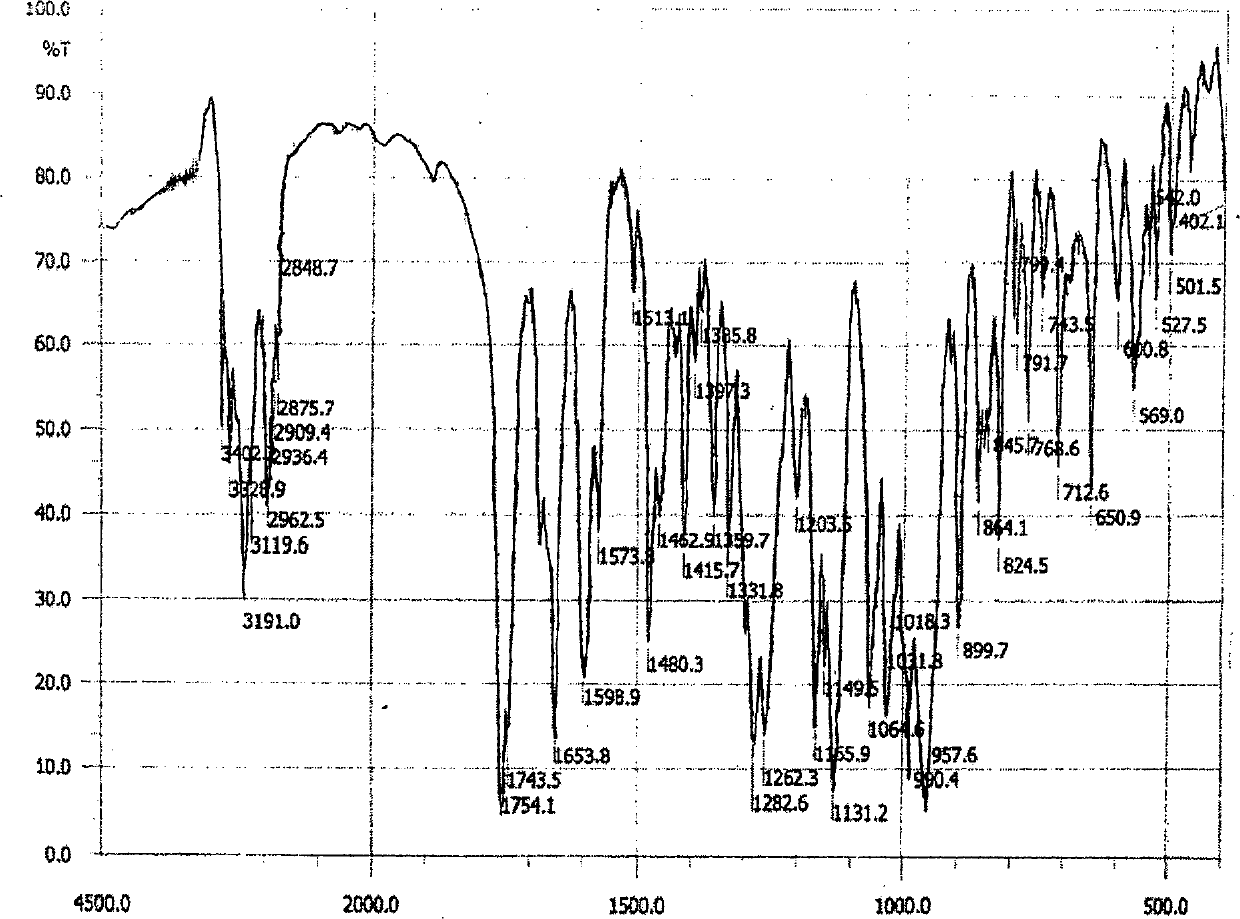
[0038] Dissolve 45g of adefovir dipivoxil crude product in 150ml of dimethyl carbonate solvent, heat to 40°C, stir to dissolve, filter, cool the filtrate to 10-11°C, crystallize for 5 hours, filter, and obtain solid at 50°C, 5Kpa After vacuum drying for 20 hours, 43.8 g of adefovir dipivoxil crystals were obtained, and the content was 99.6% as determined by HPLC. Cu-Kα radiation, X-ray powder diffraction represented by 2θ angle and interplanar spacing (d value) at about 8.4 (10.8), about 9.8 (9.0), about 14.2 (6.2), about 14.8 (6.0), about 16.2 (5.5), about 17.0(5.2), about 19.7(4.5), about 21.1(4.2), about 22.0(4.1), about 23.1(3.9), about 23.8(3.7), about 25.1(3.6), about 25.6(3.5 ), about 28.8 (3.1), about 29.8 (2.9), about 33.3 (2.6), about 34.6 (2.6), and about 38.9 (2.3) have obvious characteristic absorption peaks, see the attached figure 1 . The differential thermal analysis (DSC) spectrum has an endothermic peak at 94°C, see the attached figure 2 . Infrared absor...
Embodiment 2
[0040] Dissolve 45g of adefovir dipivoxil crude product in 150ml of diethyl carbonate solvent, heat to 40°C, stir to dissolve, filter, cool the filtrate to 14-15°C, crystallize for 5 hours, filter, and obtain solid at 50°C, 5Kpa After vacuum drying for 25 hours, 42.5 g of adefovir dipivoxil crystals were obtained, and the content was 99.7% as determined by HPLC. Cu-Kα radiation, X-ray powder diffraction represented by 2θ angle and interplanar spacing (d value) is at 8.4 (10.9), about 9.8 (9.0), about 14.2 (6.2), about 14.8 (6.0), about 16.2 ( 5.6), about 17.0 (5.2), about 19.7 (4.5), about 21.1 (4.2), about 22.0 (4.1), about 23.1 (3.9), about 23.8 (3.7), about 25.1 (3.6), about 25.6 (3.5) , about 28.8 (3.1), about 29.8 (2.9), about 33.4 (2.6), about 34.6 (2.6), and about 38.9 (2.3) have obvious characteristic absorption peaks. Differential thermal analysis (DSC) spectrum has an endothermic peak at 94°C. Infrared absorption spectrum (KBr tablet) at 3191cm -1 、3122cm -1 、175...
Embodiment 3
[0042] Dissolve 45g of crude adefovir dipivoxil in 150ml of ethyl methyl carbonate solvent, heat to 40°C, stir to dissolve, filter, cool the filtrate to 12-13°C, crystallize for 5 hours, filter, and obtain solid at 50°C, 5Kpa After vacuum drying for 25 hours, 43.2 g of adefovir dipivoxil crystals were obtained, and the content was 99.7% as determined by HPLC. Cu-Kα radiation, X-ray powder diffraction represented by 2θ angle and interplanar spacing (d value) at about 8.4 (10.8), about 9.8 (9.0), about 14.2 (6.3), about 14.8 (6.0), about 16.2 (5.5), about 17.0(5.2), about 19.7(4.5), about 21.1(4.2), about 22.0(4.1), about 23.1(3.9), about 23.8(3.7), about 25.1(3.6), about 25.6(3.5 ), about 28.8 (3.1), about 29.8 (2.9), about 33.3 (2.7), about 34.6 (2.6), and about 38.9 (2.3) have obvious characteristic absorption peaks. Differential thermal analysis (DSC) spectrum has an endothermic peak at 94°C. Infrared absorption spectrum (KBr tablet) at 3189cm -1 、3125cm -1 、1754cm -1 、...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com