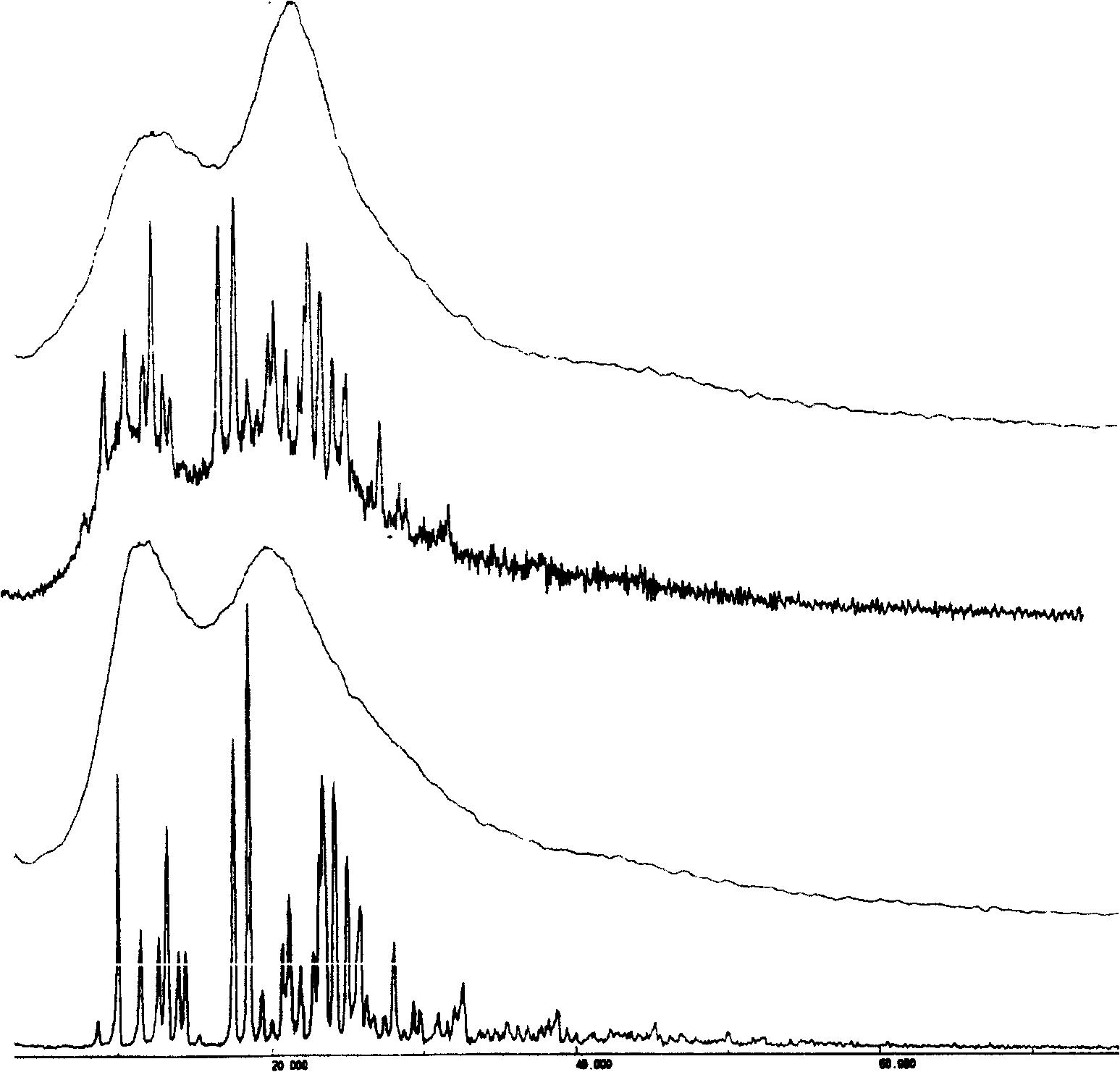
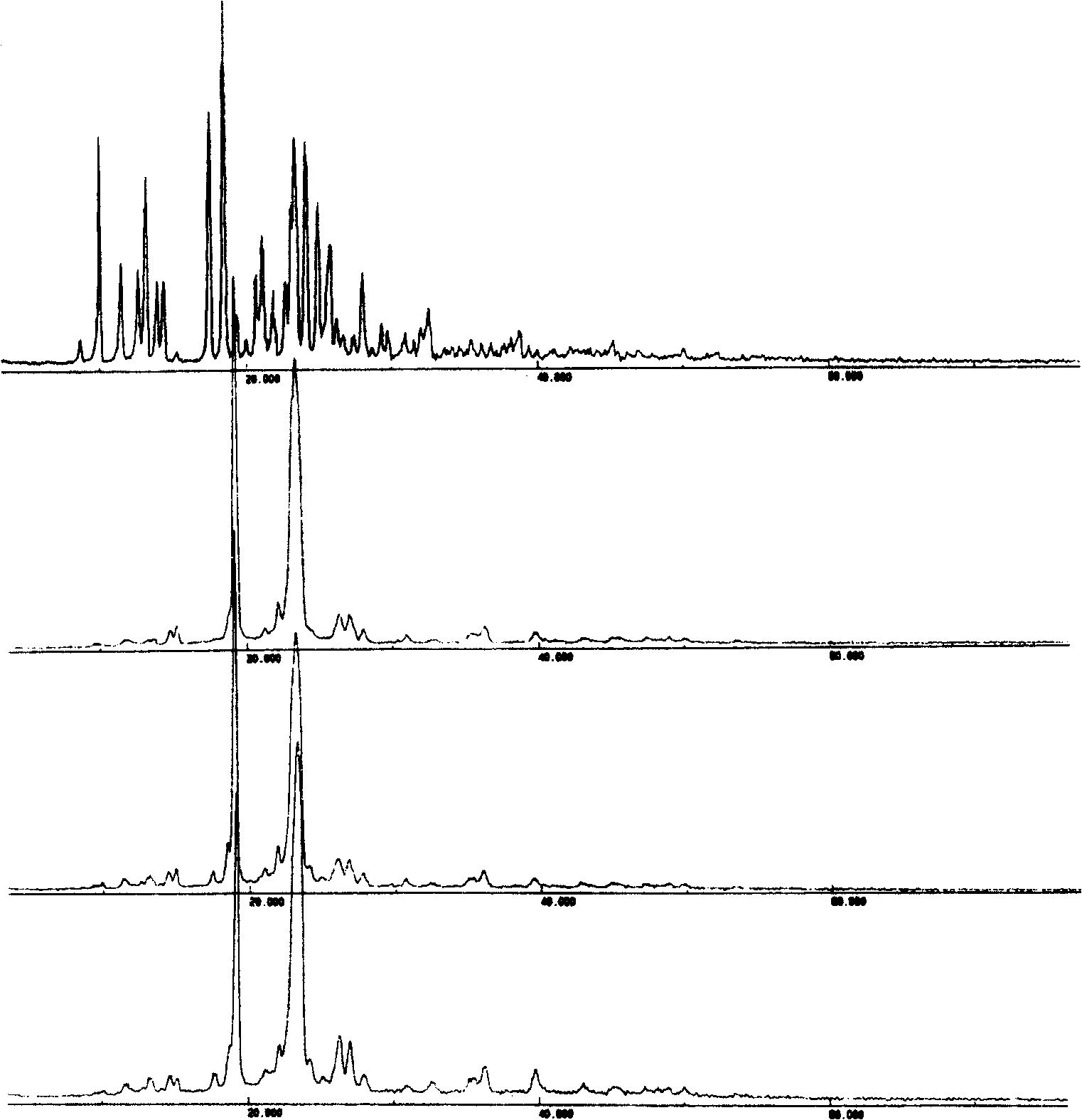
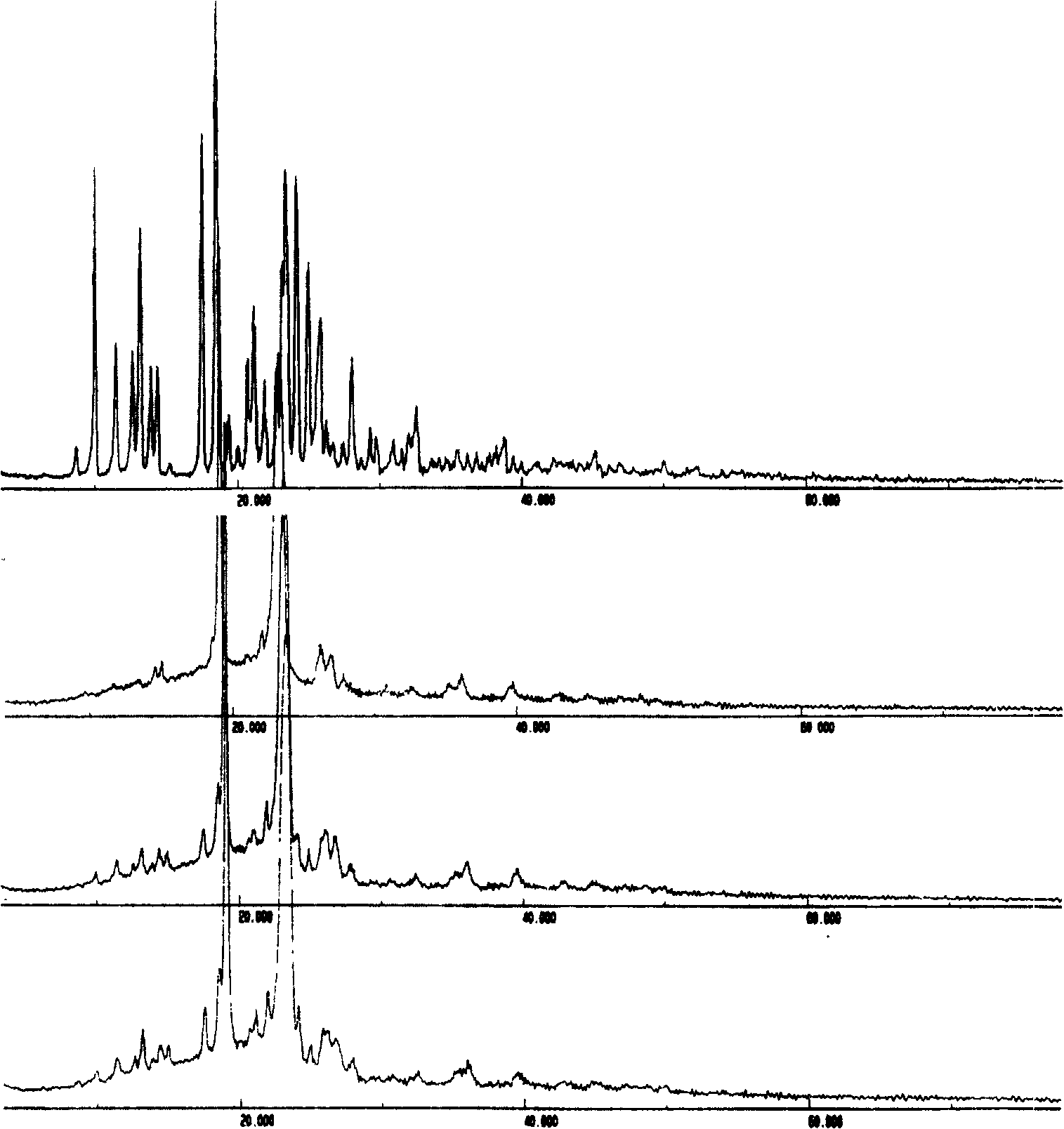
Solid bicyclic alcohol dispersion
A technology of solid dispersion and bicyclic alcohol, which is applied in the direction of organic active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as high rebound rate, lack of multi-center clinical verification, and slow dissolution rate. Effects of improved bioavailability and improved dissolution rate in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: preparation of bicyclic alcohol / PVP solid dispersion
[0059] Ratio of drug to carrier: Execute according to the table.
[0060] Table 1 drug / PVP solid dispersion feed ratio
[0061] Test No.
[0062] Preparation: Prepared by solvent method. According to the dosage in Table 1, weigh PVP and dissolve it in a certain volume of absolute ethanol, add bicyclic alcohol, and heat at 50°C to dissolve completely. Then, the solvent was removed by rotary evaporation under reduced pressure at 50°C, placed in a vacuum drying oven, and dried overnight at 40°C. The next day, it was taken out and crushed through a 60-mesh sieve to obtain four groups of bicyclic alcohol / PVP solid dispersions.
Embodiment 2
[0063] Embodiment 2: preparation of bicyclic alcohol / PVP physical mixture
[0064] Ratio of drug to carrier: Execute according to the table.
[0065] Table 2 Drug / PVP physical mixture feed ratio
[0066] Test No.
[0067] PVP volume
[0068] Preparation: Prepare directly by mixing. Pass PVP and bicyclic alcohol through 80-mesh sieve respectively, feed according to the ratio in Table 2, sieve and mix, and obtain four groups of bicyclic alcohol / PVP physical mixtures.
Embodiment 3
[0069] Embodiment 3: preparation of bicyclic alcohol / PEG solid dispersion
[0070] Ratio of drug to carrier: Execute according to the table.
[0071] Table 3 drug / PEG solid dispersion feed ratio
[0072] Test No.
[0073] Preparation: Prepared by melting method. According to the dosage in Table 3, weigh PEG8000, heat it at 70~80°C until it is completely melted, add bicyclic alcohol, stir well, continue heating until it is completely melted, quickly put it into an ice-water bath to cool and solidify, and crush it through a 60-mesh sieve to obtain Four sets of bicyclic alcohol / PEG solid dispersions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com