Red fluorescent dye and its systhesis method and use
A technology of red fluorescence and synthesis method, which is applied in the field of fluorescent dyes with red light emission and its synthesis, which can solve the problems of reducing reaction yield, raw material consumption, and increasing cost, and achieve high yield, few reaction steps, and good red color Effects of Chromaticity Properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
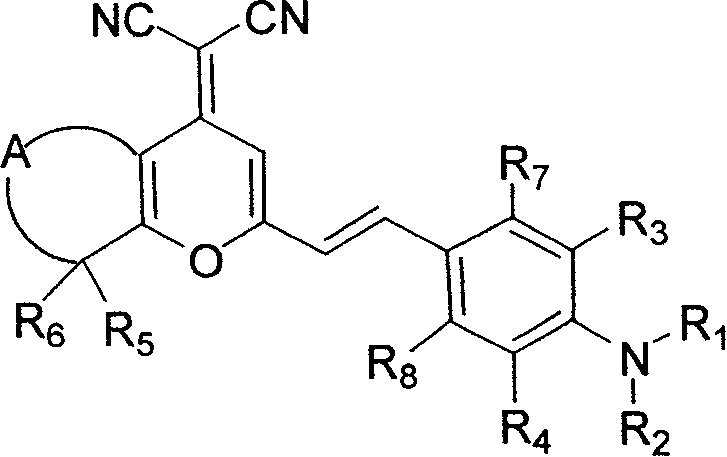
[0084] Example 1: 4-Dicyanomethenyl-8-methyl-2-(4-dimethylaminostyryl)-5,6,7,8-tetrahydro-4H-1-benzopyran
[0085] (1). Synthesis of 1-(4-morpholine)-6-methyl-cyclohexene
[0086]
[0087] Add 11.2g (0.1mol) of 2-methylcyclohexanone, 14g (0.12mol) of morpholine, 200ml of toluene, and 1g (0.006mol) of p-toluenesulfonic acid into a round bottom flask, and heat to reflux to separate water for 5 hours. The solvent was distilled off, and 14.5 g of the product 1-(4-morpholine)-6-methyl-cyclohexene was collected by vacuum distillation at 100-102° C. / 6 mmHg, with a yield of 78%.
[0088] (2). Synthesis of 2,8-dimethyl-5,6,7,8-tetrahydro-4H-1-benzopyran-4-one
[0089]
[0090] Add 18.1g (0.1mol) 1-(4-morpholine)-6-methyl-cyclohexene, 28.5g (0.2mol) 2,2,6-trimethyl-1,3- Dioxin-4-one was heated to reflux at 150°C for 8 hours. Cool, add 500ml chloroform, wash three times with 100ml 5%-10% sodium hydroxide aqueous solution, then wash three times with 100ml water, dry with anhydrou...
Embodiment 2
[0103] Example 2: 4-Dicyanomethenyl-8-methyl-2-(4-dimethylaminostyryl)-5,6,7,8-tetrahydro-4H-1-benzopyran
[0104] (1). The synthetic method of 1-(4-morpholine)-6-methyl-cyclohexene is the same as step (1) in Example 1
[0105] (2). Synthesis of 4-dicyanomethenyl-2,8-dimethyl-5,6,7,8-tetrahydro-4H-1-benzopyran
[0106]
[0107] Add 18.1g (0.1mol) 1-(4-morpholine)-6-methyl-cyclohexene, 28.5g (0.2mol) 2,2,6-trimethyl-1,3- Dioxin-4-one was heated to reflux at 150°C for 8 hours. Cool, add 500ml chloroform, wash three times with 150ml 5%-10% aqueous sodium hydroxide solution, then wash three times with 100ml water, dry with anhydrous sodium sulfate, distill off chloroform, then add 13.2g (0.2mol) malononitrile, 150ml Ethanol, 15ml catalyst (5ml hexahydropyridine + 15ml acetic acid), heated to reflux for 2 hours. The solvent was distilled off under reduced pressure, cooled, the solid was washed with water, and dried. The solid was recrystallized with chloroform / n-hexane to obt...
Embodiment 3
[0116] Example 3: 4-Dicyanomethenyl-8-methyl-2-(juloridine-9-vinyl)-5,6,7,8-tetrahydro-4H-1-benzopyran
[0117] (1). The synthetic method of 1-(4-morpholine)-6-methyl-cyclohexene is the same as step (1) in Example 2
[0118](2).4-dicyanomethenyl-2,8-dimethyl-5,6,7,8-tetrahydro-4H-1-benzopyran synthetic method and steps in Example 2 ( 2) the same, wherein the catalyst is changed to 15ml hexahydropyridine
[0119] (3).4-Dicyanomethenyl-8-methyl-2-(juloridine-9-vinyl)-5,6,7,8-tetrahydro-4H-1-benzopyran
[0120]
[0121] In a round bottom flask, add 0.23g (1mmol) 4-dicyanomethenyl-2,8-dimethyl-5,6,7,8-tetrahydro-4H-1-benzopyran, 0.22g ( 1.1mmol) of 9-formyl juloridine, 15ml of acetonitrile, 0.10ml of hexahydropyridine, and heated to reflux for 20 hours. Acetonitrile was distilled off, cooled, the solid was rinsed with acetonitrile several times, and dried to obtain 0.29 g of the product, with a yield of 71%.
[0122] NMR 1 H NMR (CDCl 3 )δ(ppm): 1.38(d, 3H), 1.5-1.9(m, 8H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com