Montelukast oral disintegrating tablet formulation and its preparing method
A technology of orally disintegrating tablets and montelukast sodium, which is applied in the field of preparation of orally disintegrating tablet preparations of montelukast, can solve problems such as poor physical strength of orally disintegrating tablets, unsuitability for industrial production, and insoluble tablets , to achieve the effect of accelerating the dissolution of the main drug, the process is feasible, and the drug release is fast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
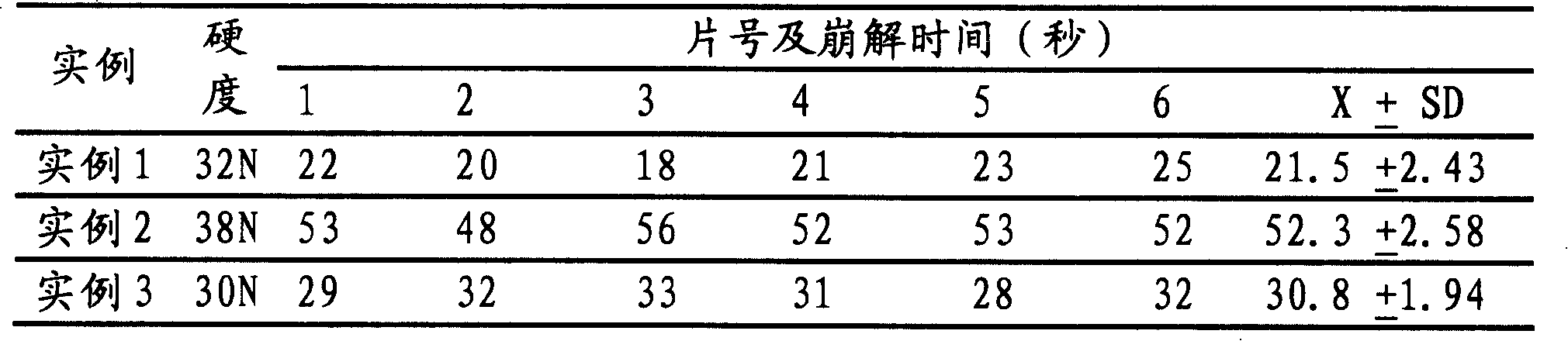
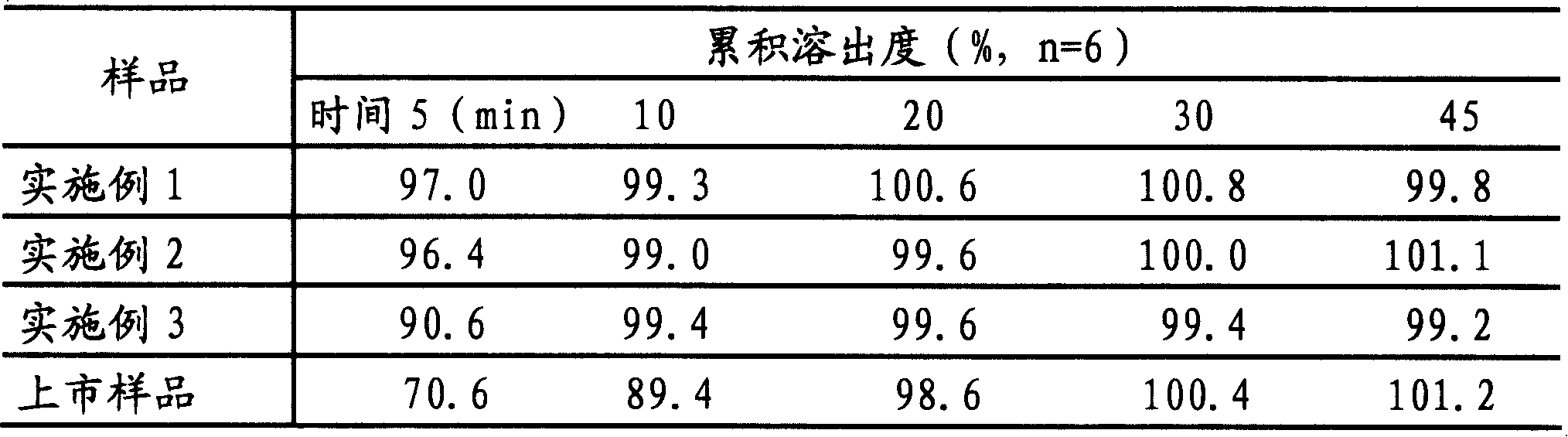
Embodiment 1
[0032] Preparation prescription of the present invention is made up of following components by weight
[0033] Montelukast Sodium (Containing Montelukast 40g) 41.6g
[0034] Mannitol (Pearlitol SD100) 1500g
[0035] Hydroxypropyl Methyl Cellulose E5 80g
[0036] Purified water 800g
[0037] Croscarmellose Sodium 100g
[0038] Micronized silica gel 10g
[0039] Solid lemon essence 4g
[0041] A total of 10,000 pieces were made
[0042] Process: Feed the prescribed amount of mannitol into the fluidized bed equipped with bottom spray equipment as the base and in a fluidized state, dissolve the prescribed amount of montelukast, stabilizer, hydroxypropyl methylcellulose and micropowdered silica gel Or dispersed in purified water, sprayed on the mannitol on the base to form a particle package:
[0043] Air intake volume: about 13scfm
[0044] Inlet air temperature: about 65°C
[0045] Intake air dew point: about 15°C
[0046] Atomizing air ...
Embodiment 2
[0055] Preparation prescription of the present invention is made up of following components by weight
[0056] Montelukast Sodium (Containing Montelukast 50g) 52g
[0057] Mannitol 1700g
[0058] Microcrystalline Cellulose 300g
[0059] Polyvinylpyrrolidone 50g
[0060] Purified water 500g
[0061] Croscarmellose Sodium 100g
[0062] Orange Oil Flavor 3g
[0063] Micronized silica gel 10g
[0065] A total of 10,000 pieces were made
[0066] Process: put montelukast sodium, mannitol pulverization and microcrystalline cellulose in a high-speed stirring granulator, mix evenly, dissolve polyvinylpyrrolidone in pure water, add a high-speed stirring granulator to granulate, dry, take out. Add orange oil essence, micronized silica gel and magnesium stearate, mix well, and press into tablets to obtain the product.
Embodiment 3
[0068] Preparation prescription of the present invention is made up of following components by weight
[0069] Montelukast Sodium (Containing Montelukast 100g) 104g
[0070] Mannitol (Pearlitol SD100) 2000g
[0071] Microcrystalline Cellulose 300g
[0072] Cross-linked polyvinylpyrrolidone 100g
[0073] Strawberry essence 5g
[0074] Micronized silica gel 20g
[0076] A total of 10,000 pieces were made
[0077] Process: Mix montelukast sodium, mannitol, microcrystalline cellulose, cross-linked polyvinylpyrrolidone, strawberry essence, micropowder silica gel, magnesium stearate, and press into tablets to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com