Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Functional residual capacity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
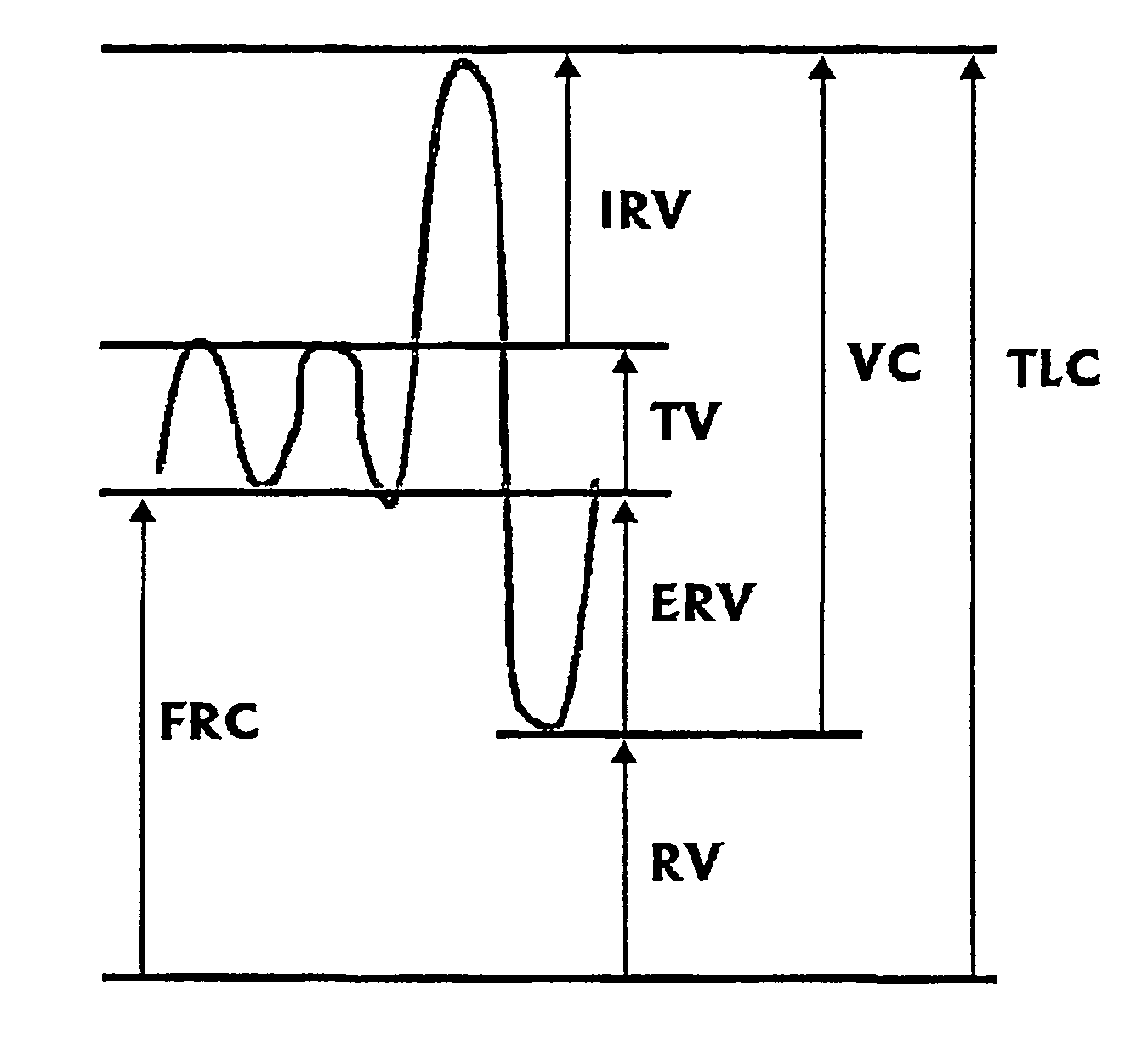
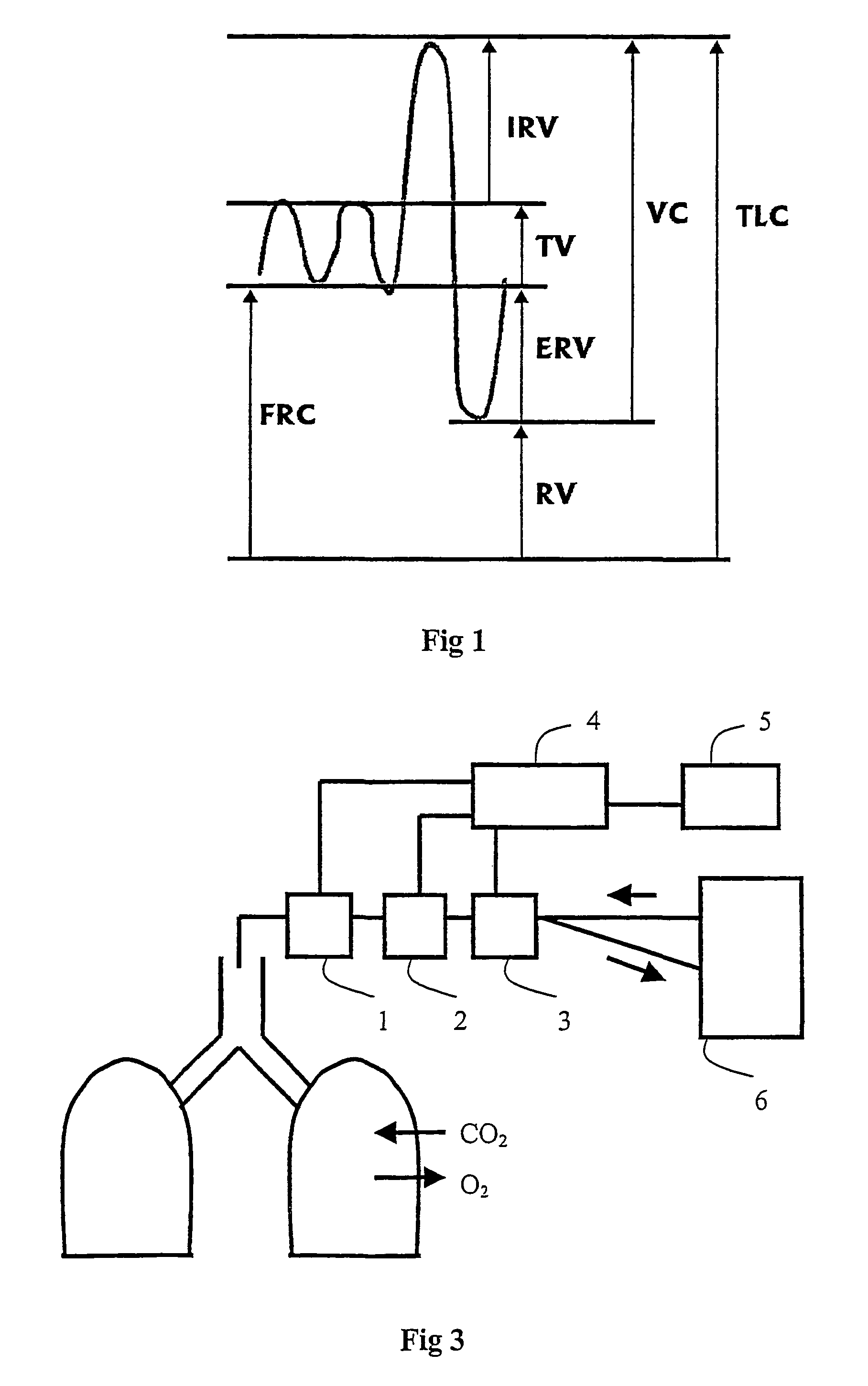
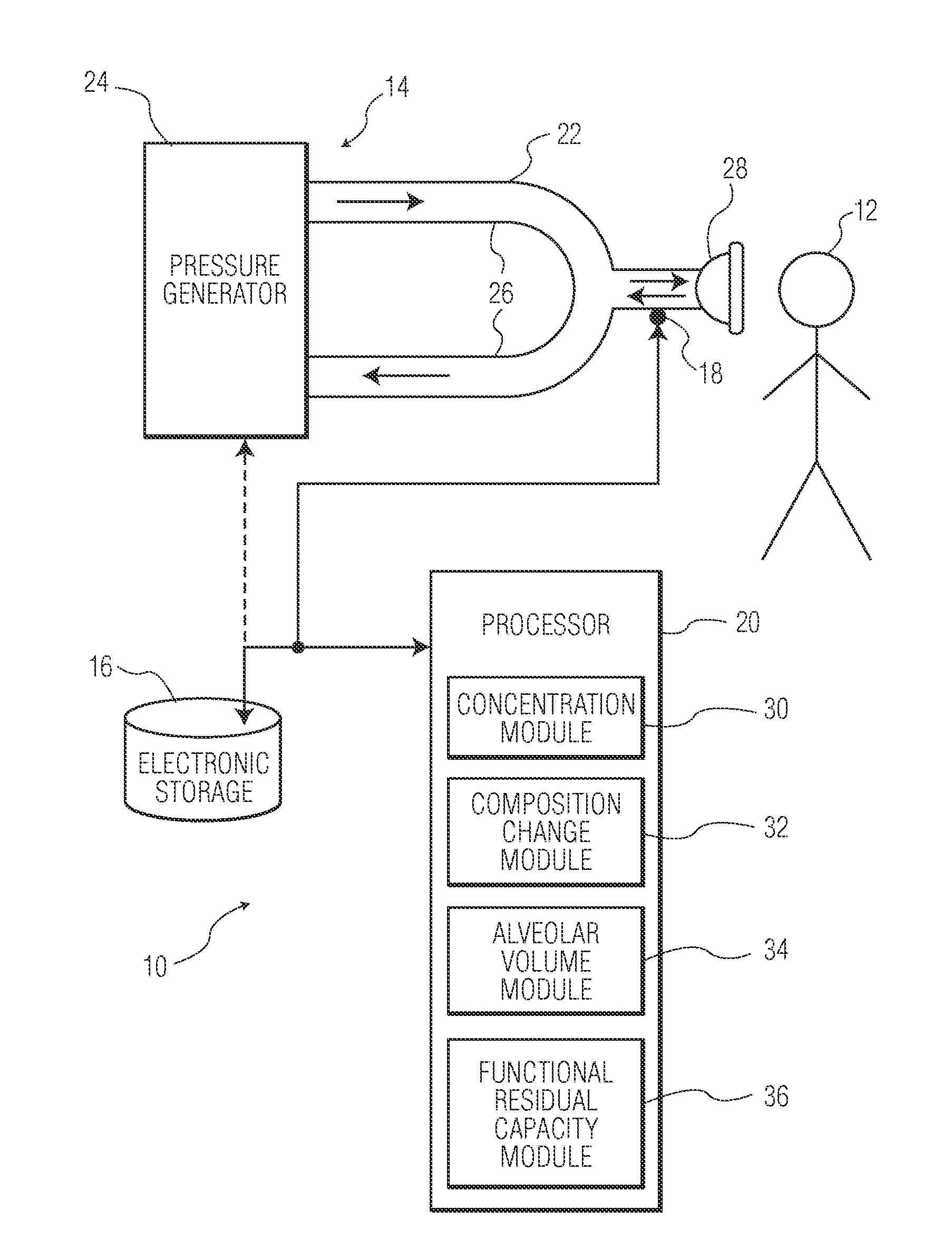
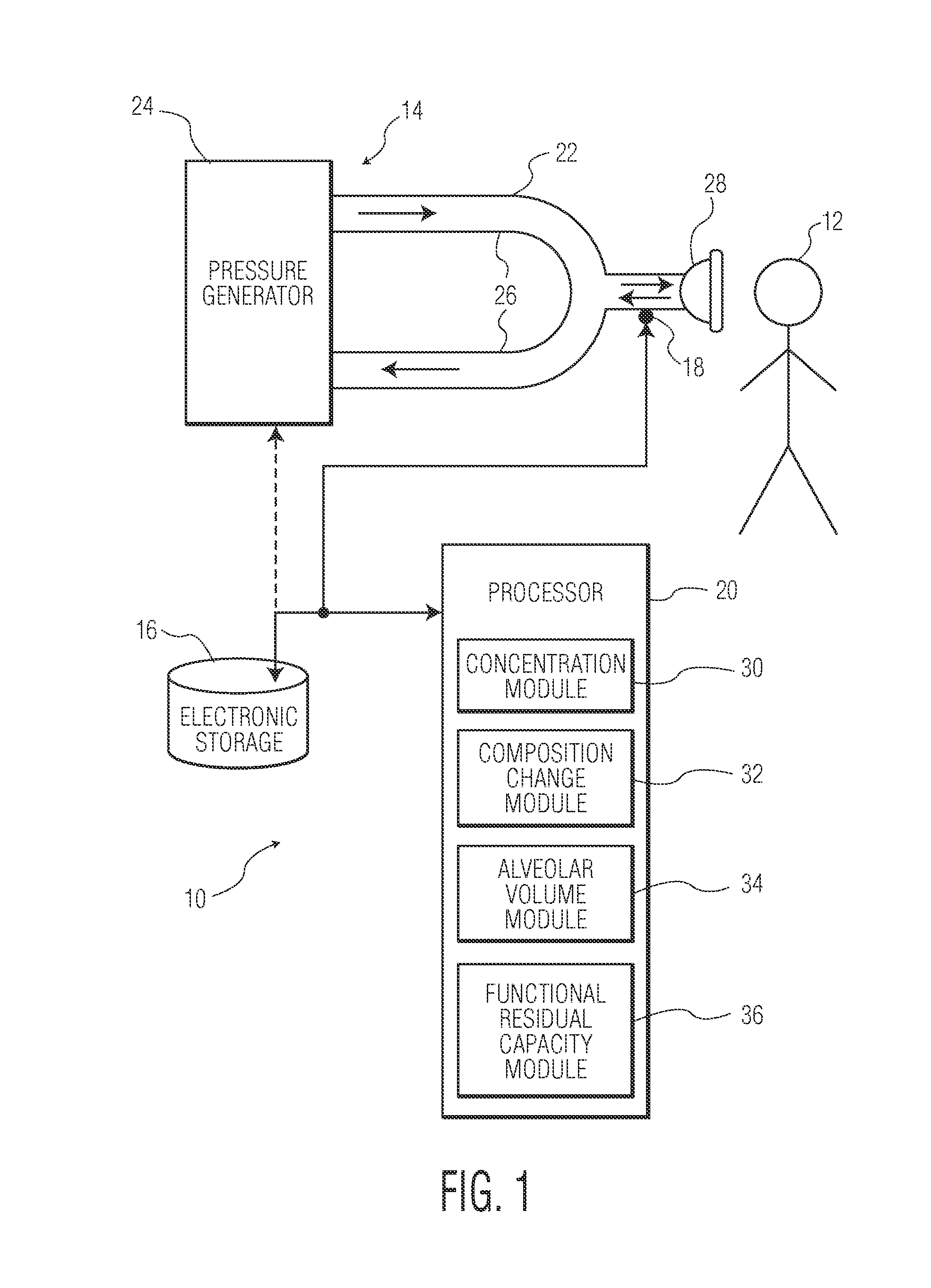
Functional Residual Capacity (FRC) is the volume of air present in the lungs at the end of passive expiration. At FRC, the opposing elastic recoil forces of the lungs and chest wall are in equilibrium and there is no exertion by the diaphragm or other respiratory muscles.
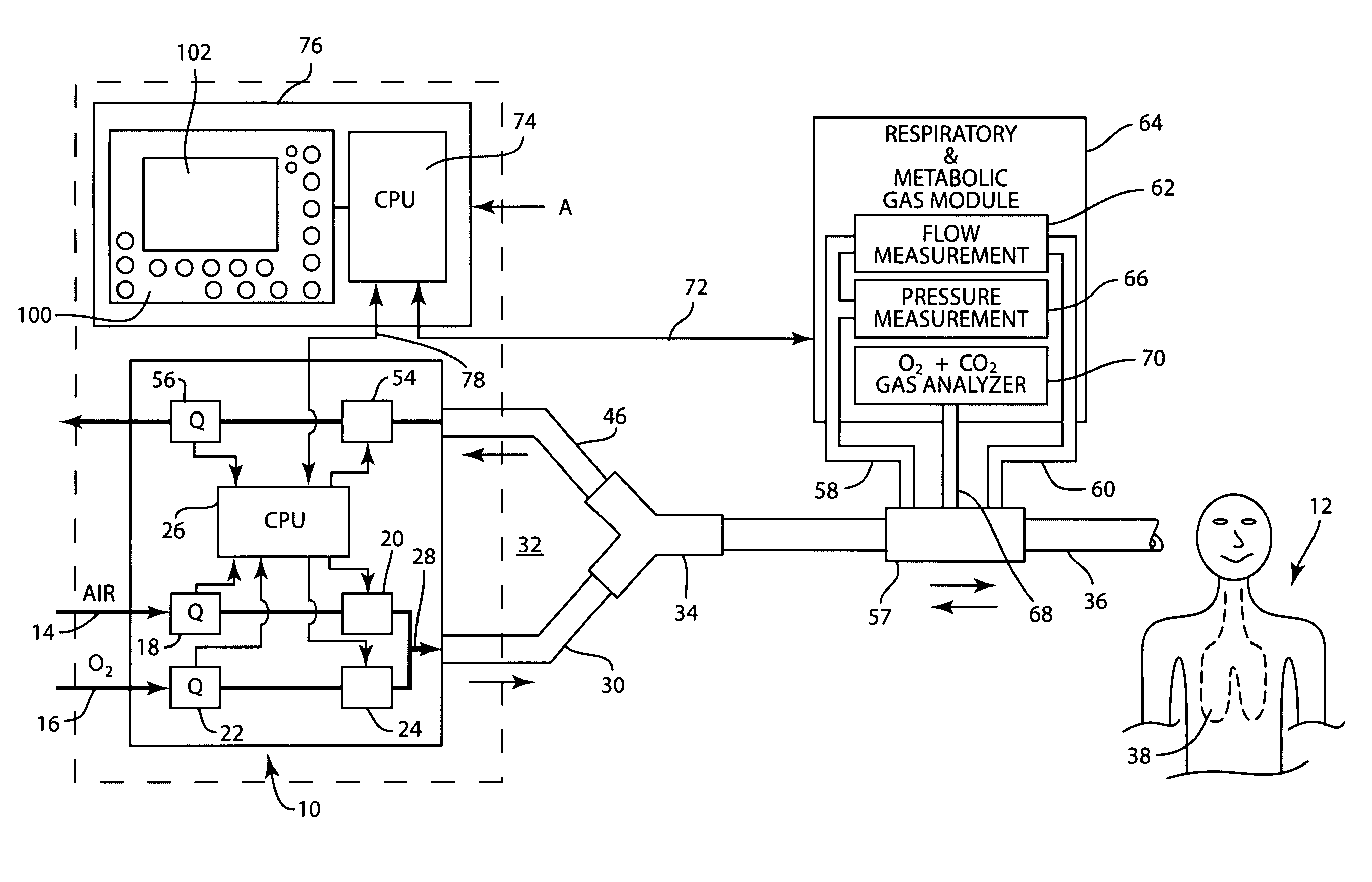
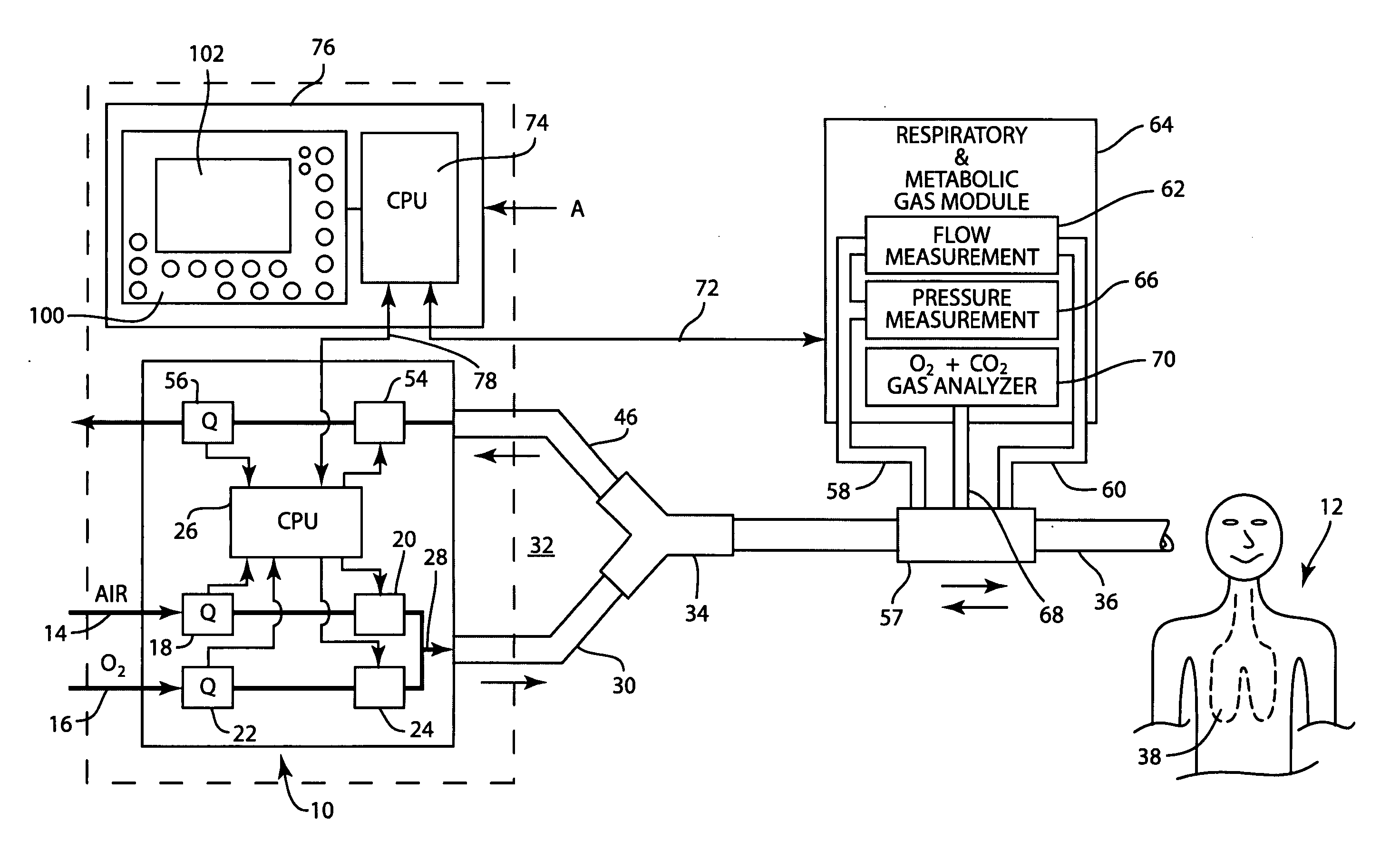
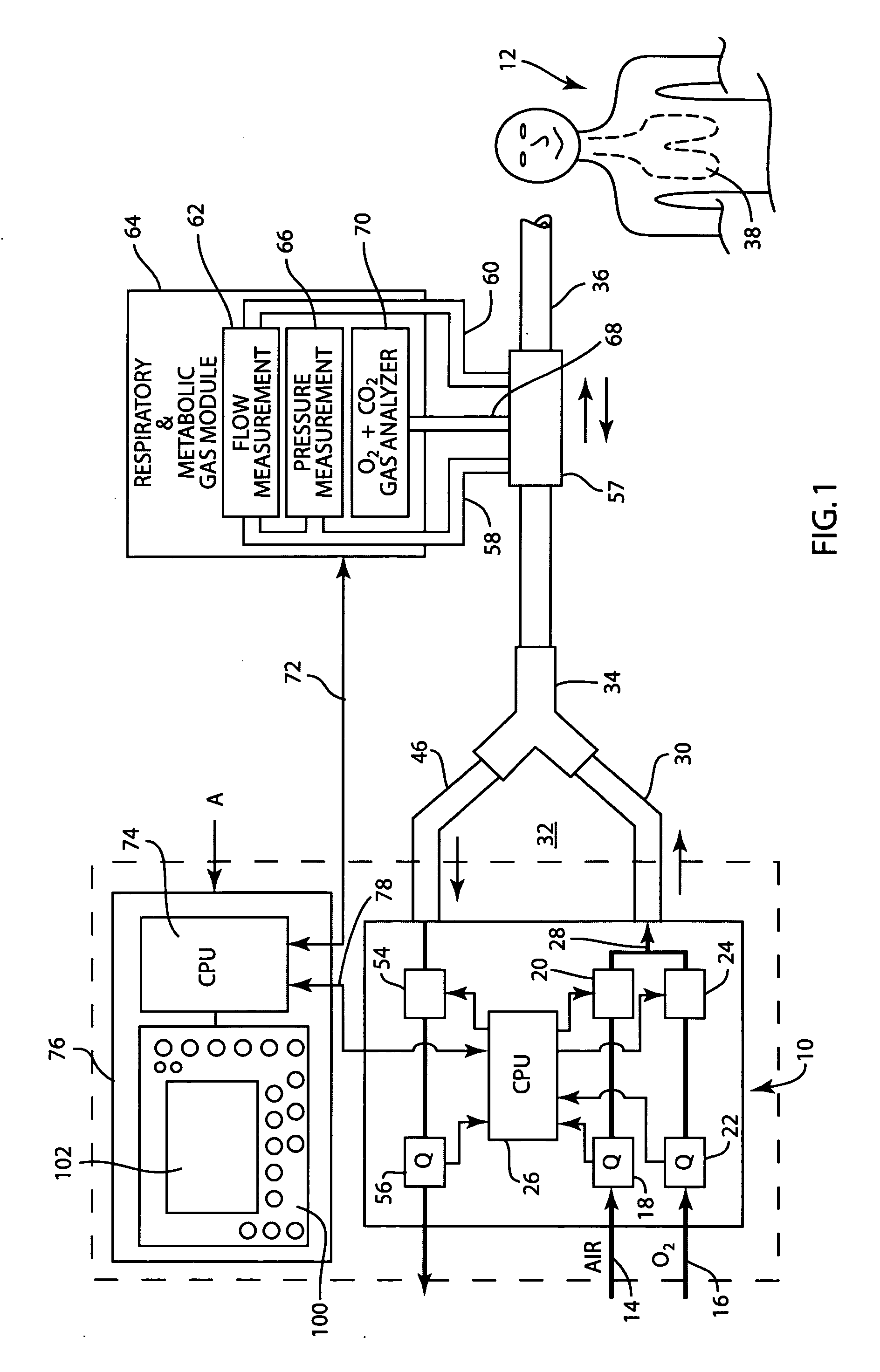
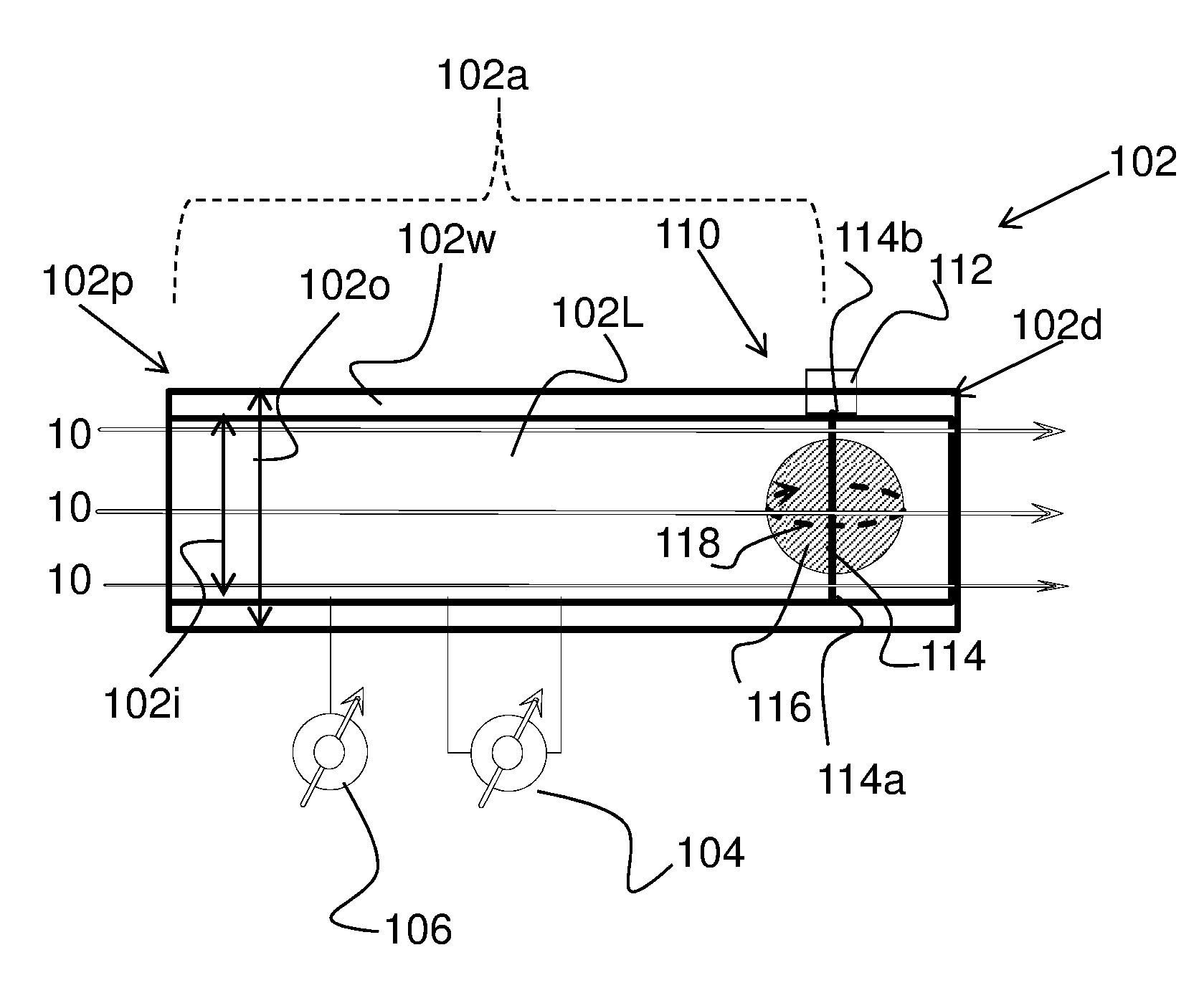
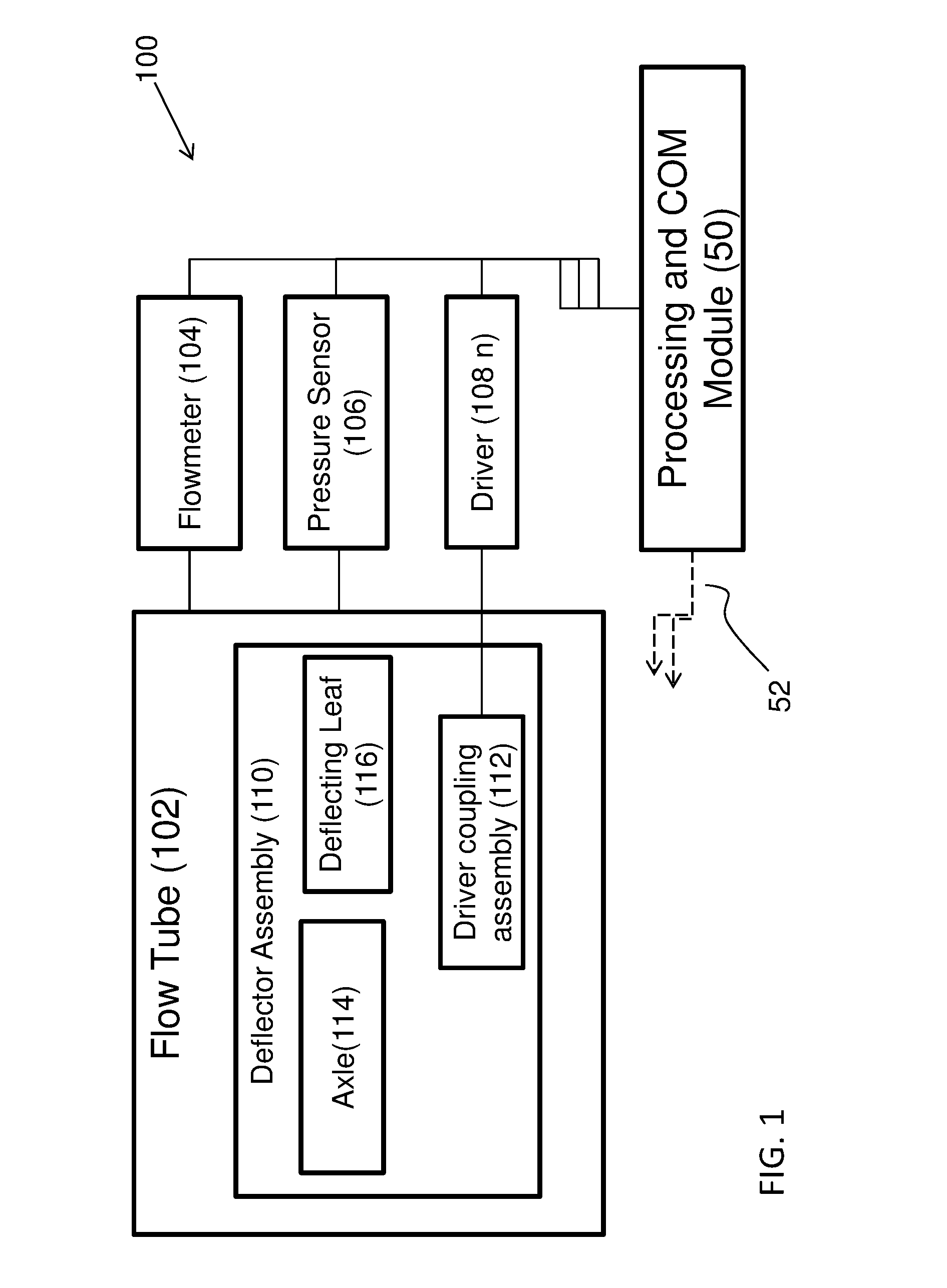
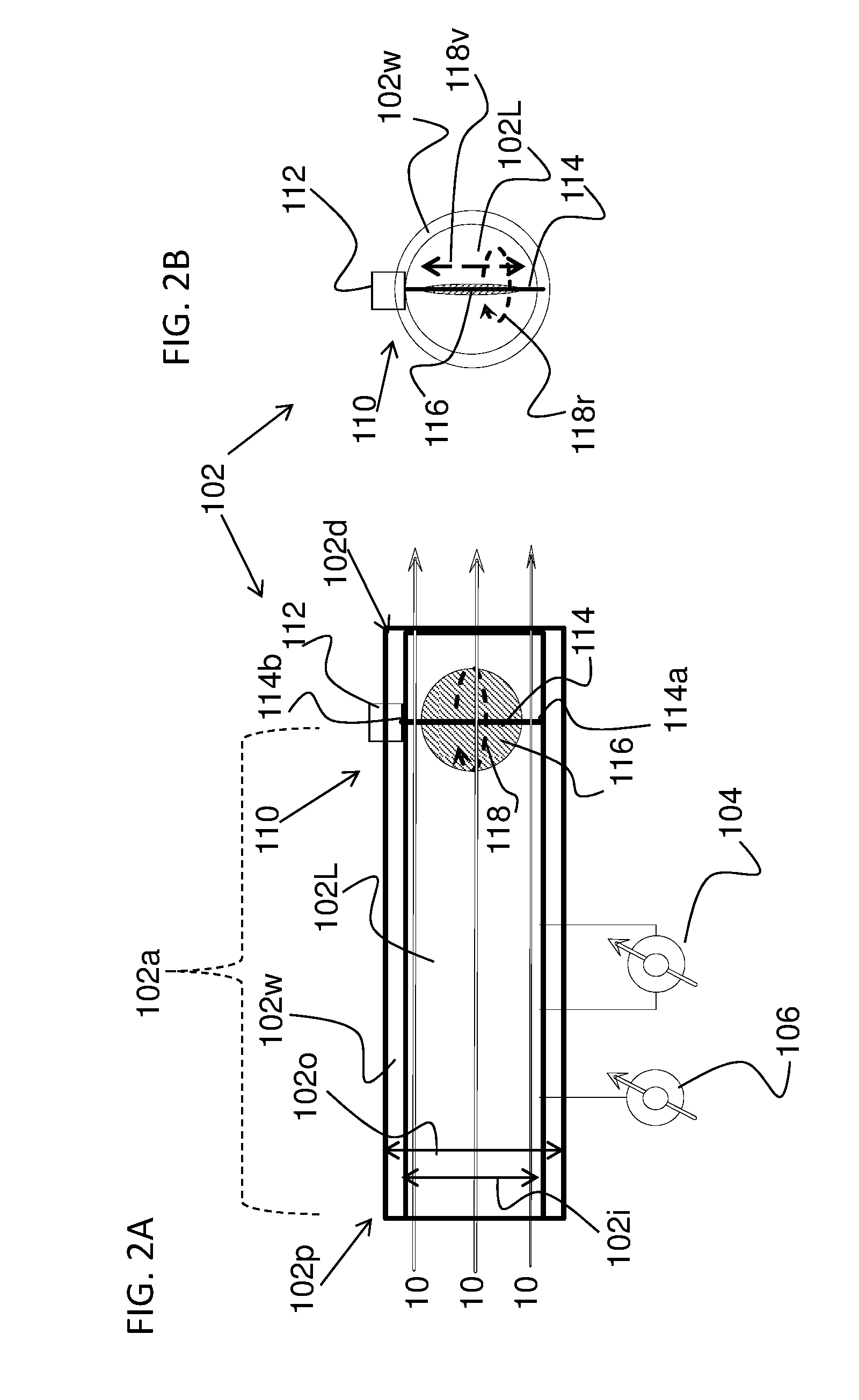
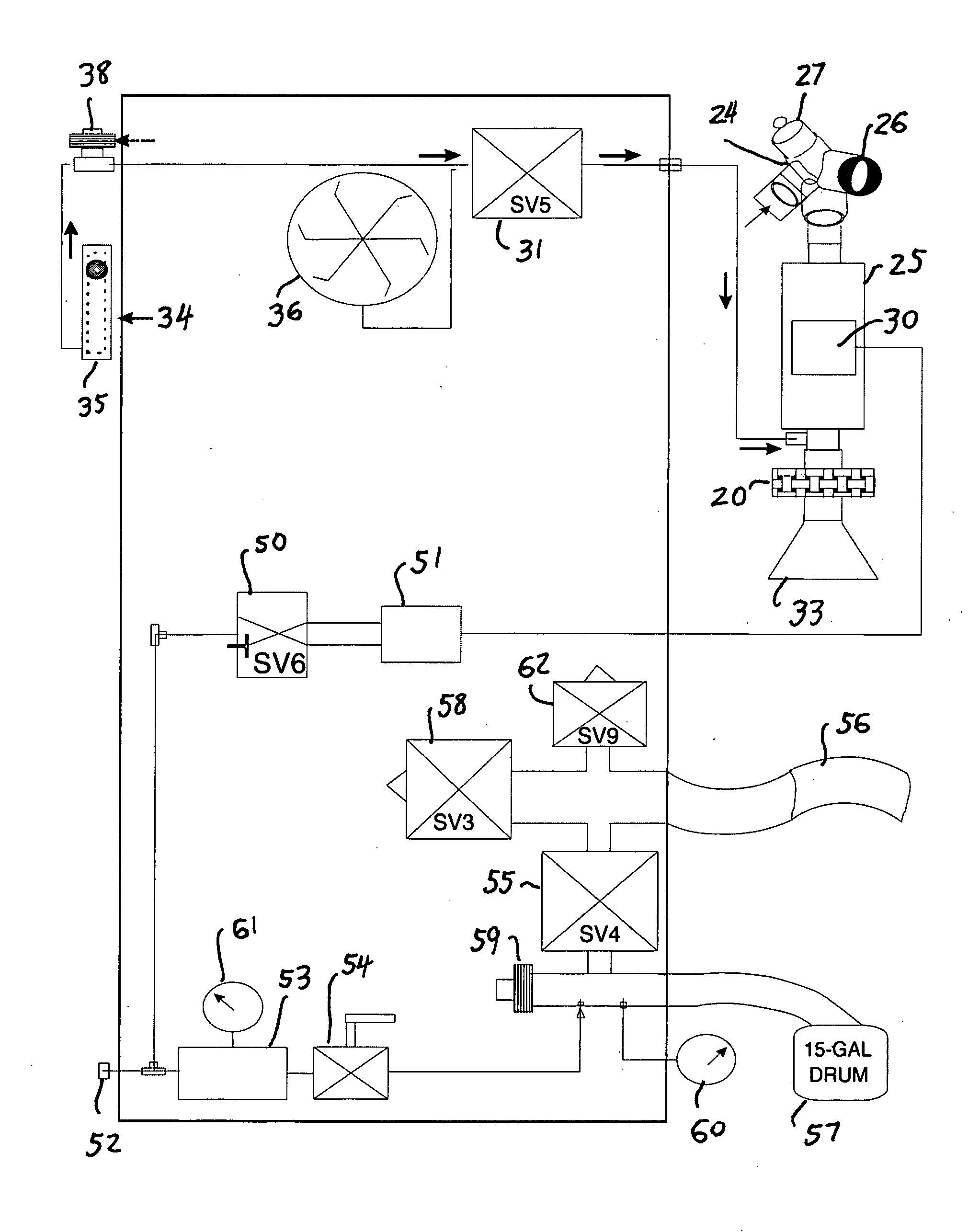
Method and apparatus for airway compensation control
ActiveUS20080091117A1Capacity remains stableRespiratorsOperating means/releasing devices for valvesForced expiratory vital capacityPulmonary compliance
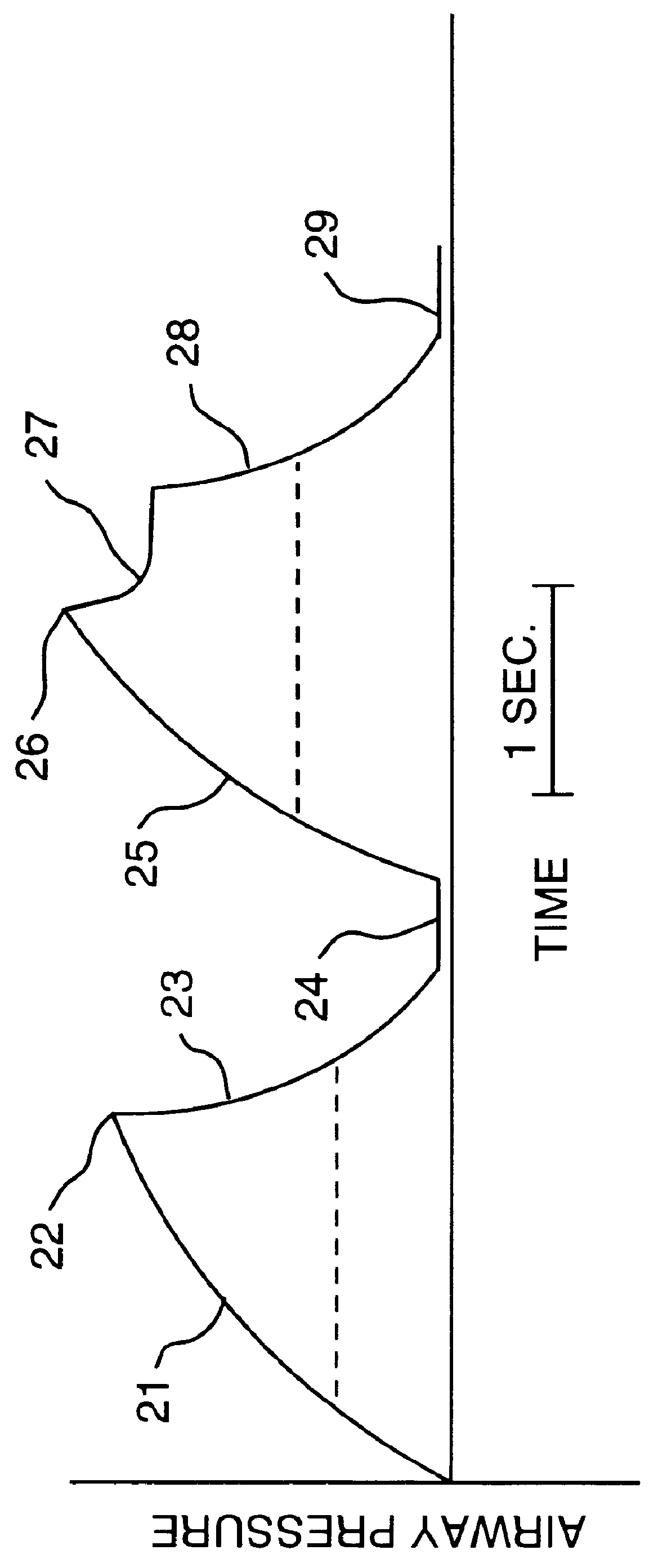
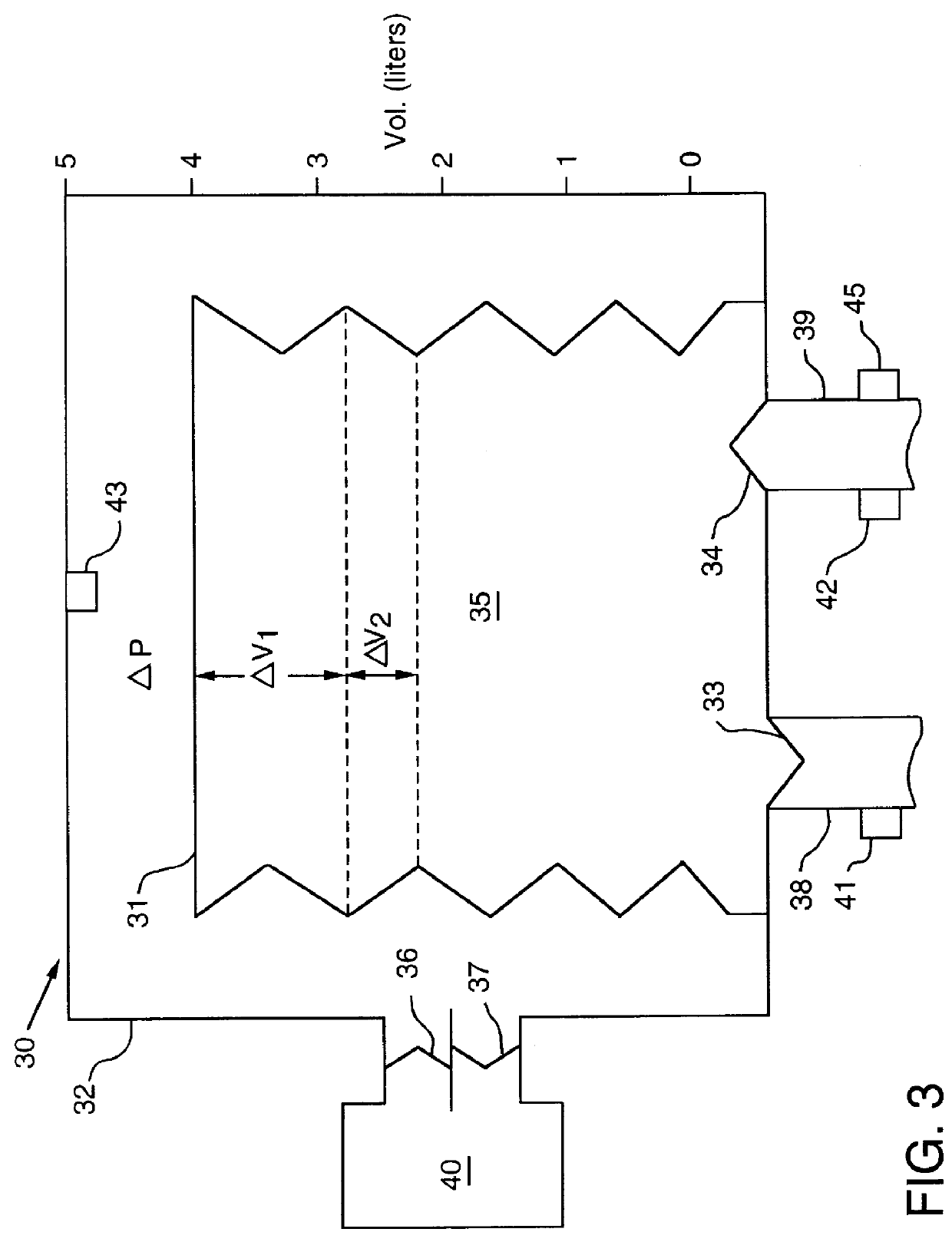
A ventilator for ventilating a patient also assists a clinician in determining a suitable PEEP for the patient. For this purpose, a graph or tabular display of a series of different value PEEPs and corresponding functional residual capacities of the patient may be provided. Or, the relationship between lung compliance and a series of different values of PEEP may be provided. Or, the amount of the lung volume recruited / de-recruited at various levels of PEEP may be determined for use in selecting a desired PEEP. To this end, the functional residual capacity of the lungs is determined for a first PEEP level. The PEEP is then altered to a second level and a spirometry dynostatic curve of lung volume and pressure data is obtained. The lung volume on the dynostatic curve at a lung pressure corresponding to the first PEEP value is obtained. The difference between the functional residual capacity of the lungs at the first PEEP level and that determined from the dynostatic curve represents the lung volume recruited / de-recruited when changing between said first and second PEEPs.
Owner:GENERAL ELECTRIC CO
Apparatus and method for identifying optimal PEEP
InactiveUS20070062532A1Quickly and easily determineSure easyRespiratorsOperating means/releasing devices for valvesForced expiratory vital capacityRadiology
A ventilator for ventilating a patient also assists a clinician in determining a suitable PEEP for the patient. For this purpose, a graph or tabular display of a series of different value PEEPs and corresponding functional residual capacities of the patient may be provided. Or, the amount of the lung volume recruited / de-recruited at various levels of PEEP may be determined for use in selecting a desired PEEP. To this end, the functional residual capacity of the lungs is determined for a first PEEP level. The PEEP is then altered to a second level and a spirometry dynostatic curve of lung volume and pressure data is obtained. The lung volume on the dynostatic curve at a lung pressure corresponding to the first PEEP value is obtained. The difference between the functional residual capacity of the lungs at the first PEEP level and that determined from the dynostatic curve represents the lung volume recruited / de-recruited when changing between said first and second PEEPs.
Owner:GENERAL ELECTRIC CO
Apparatus and method for identifying FRC and PEEP characteristics
InactiveUS20070062533A1Quickly and easily determineSure easyRespiratorsOperating means/releasing devices for valvesForced expiratory vital capacityPulmonary compliance
A ventilator for ventilating a patient also assists a clinician in determining a suitable PEEP for the patient. For this purpose, a graph or tabular display of a series of different value PEEPs and corresponding functional residual capacities of the patient may be provided. Or, the relationship between lung compliance and a series of different values of PEEP may be provided. Or, the amount of the lung volume recruited / de-recruited at various levels of PEEP may be determined for use in selecting a desired PEEP. To this end, the functional residual capacity of the lungs is determined for a first PEEP level. The PEEP is then altered to a second level and a spirometry dynostatic curve of lung volume and pressure data is obtained. The lung volume on the dynostatic curve at a lung pressure corresponding to the first PEEP value is obtained. The difference between the functional residual capacity of the lungs at the first PEEP level and that determined from the dynostatic curve represents the lung volume recruited / de-recruited when changing between said first and second PEEPs.
Owner:GENERAL ELECTRIC CO
Apparatus and method for determining and displaying functional residual capacity data and related parameters of ventilated patients
ActiveUS7530353B2Tracheal tubesOperating means/releasing devices for valvesFRC - Functional residual capacityRadiology
A ventilator for ventilating a patient has means integrated therewith for carrying out a determination of the functional residual capacity of the patient using an inert gas wash in / wash out technique. To this end, the ventilator operates to alter the inert gas content of breathing gases provided to the patient. The amount of inert gas expired by the patient is obtained and used to determine functional residual capacity on a breath-by-breath basis. A graph of the functional residual capacities for a given number of breaths is produced. Thereafter, the inert gas levels in the breathing gases are returned to the original levels and further functional residual capacity determinations and a graph of same provided. The functional residual capacity information may also be provided in tabular form. A log of functional residual capacity determinations and ventilator settings or patient treatments affecting same may also be provided.
Owner:GENERAL ELECTRIC CO
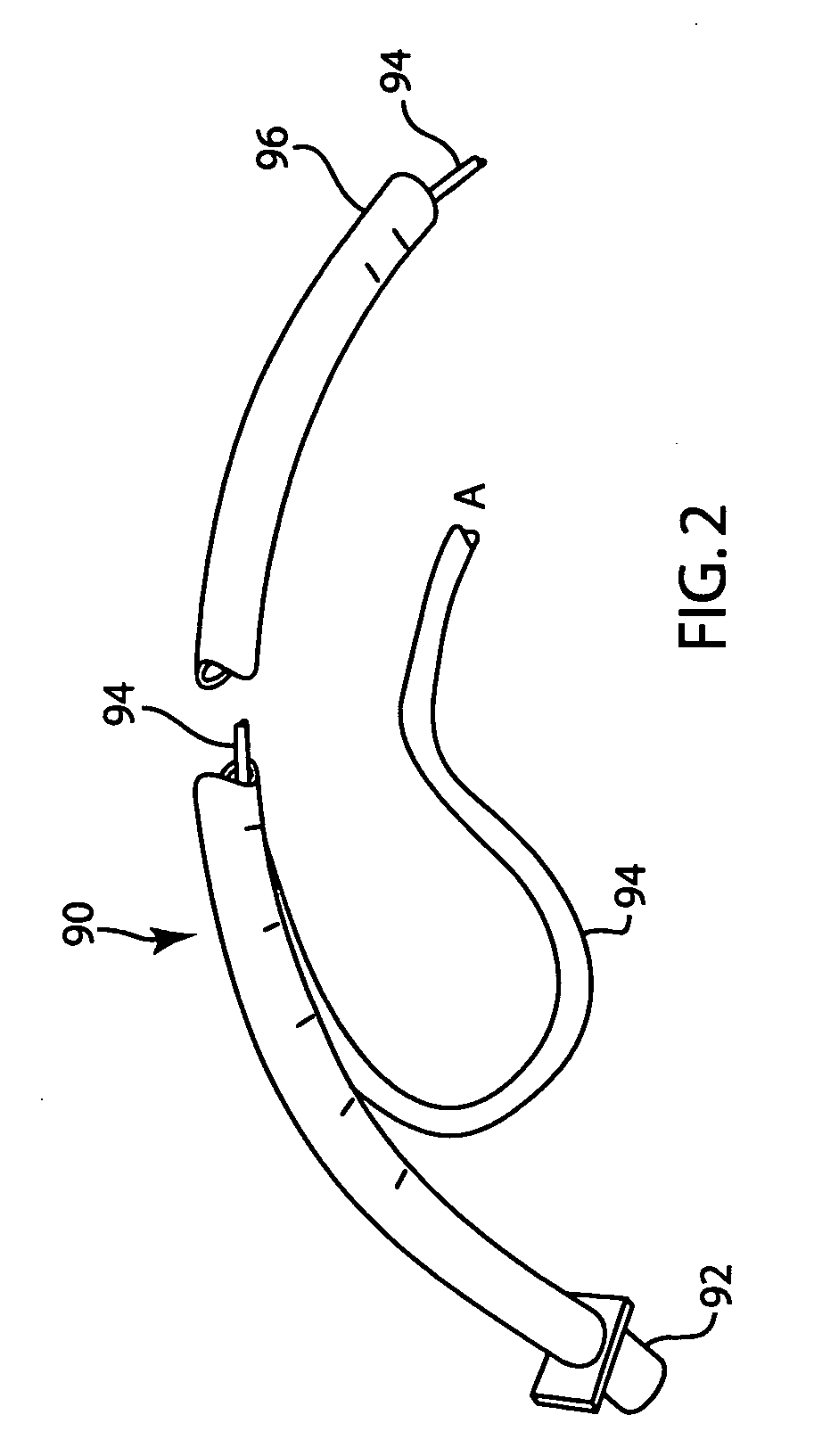
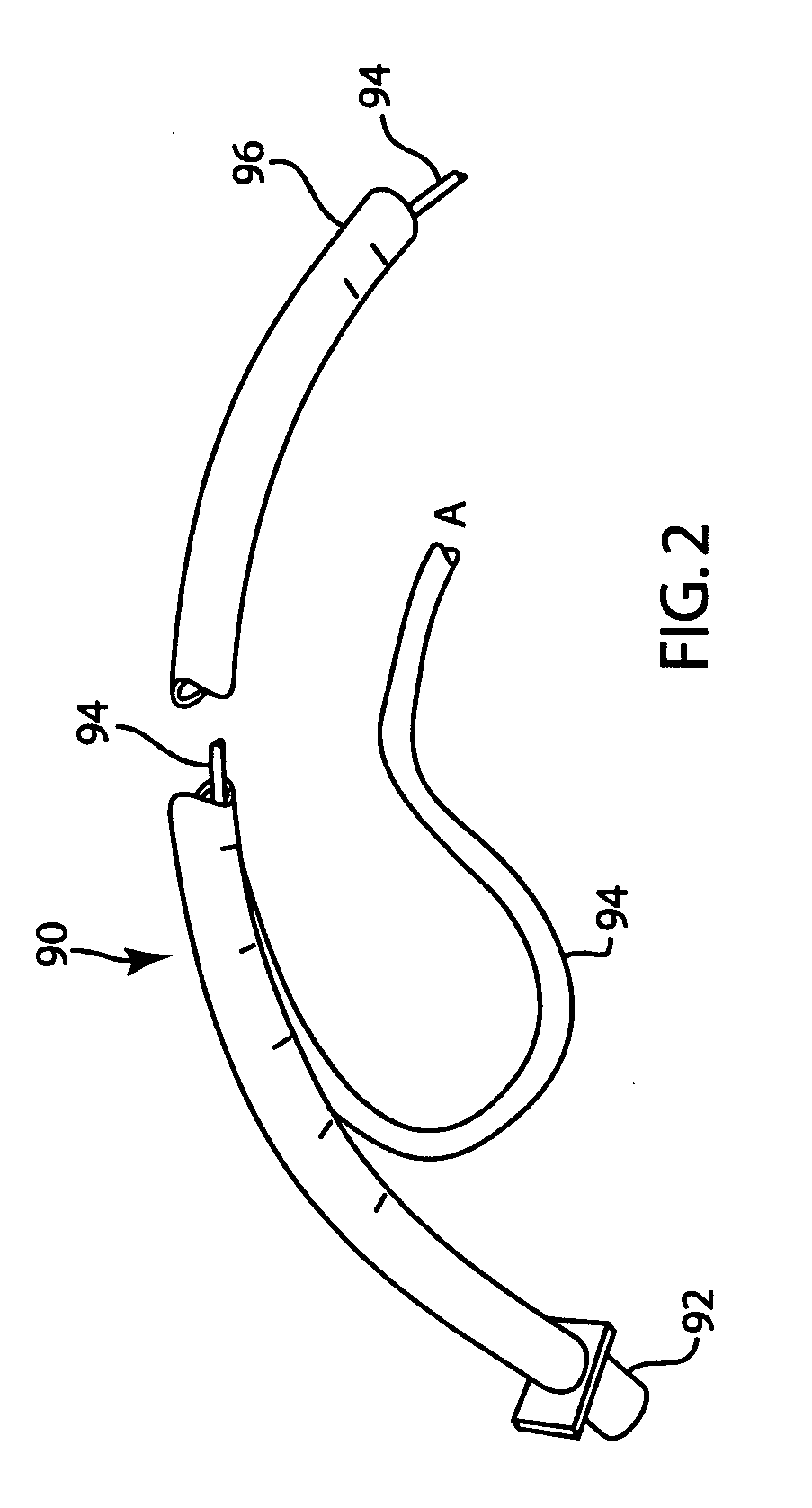
Lung volume monitoring method and device
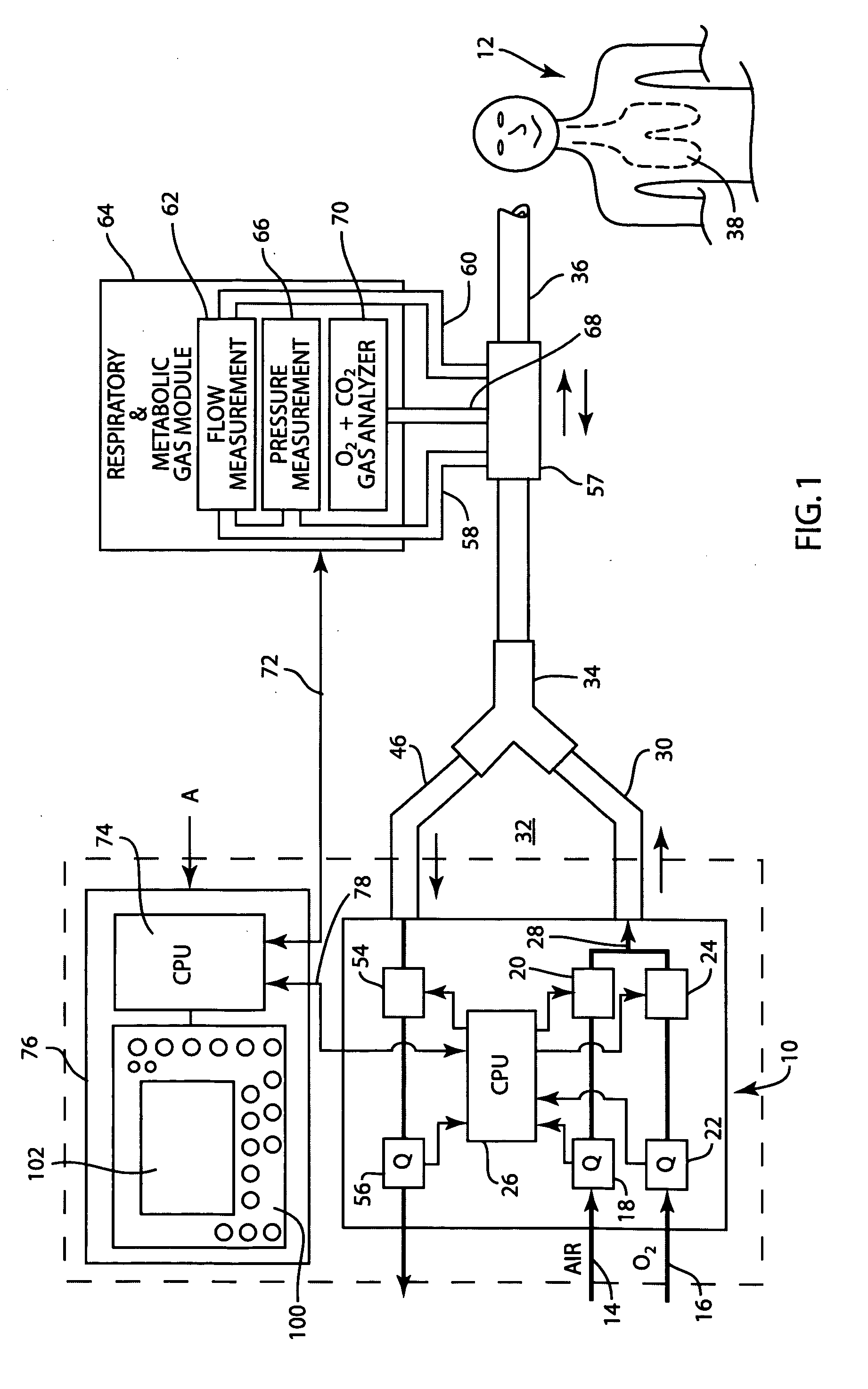
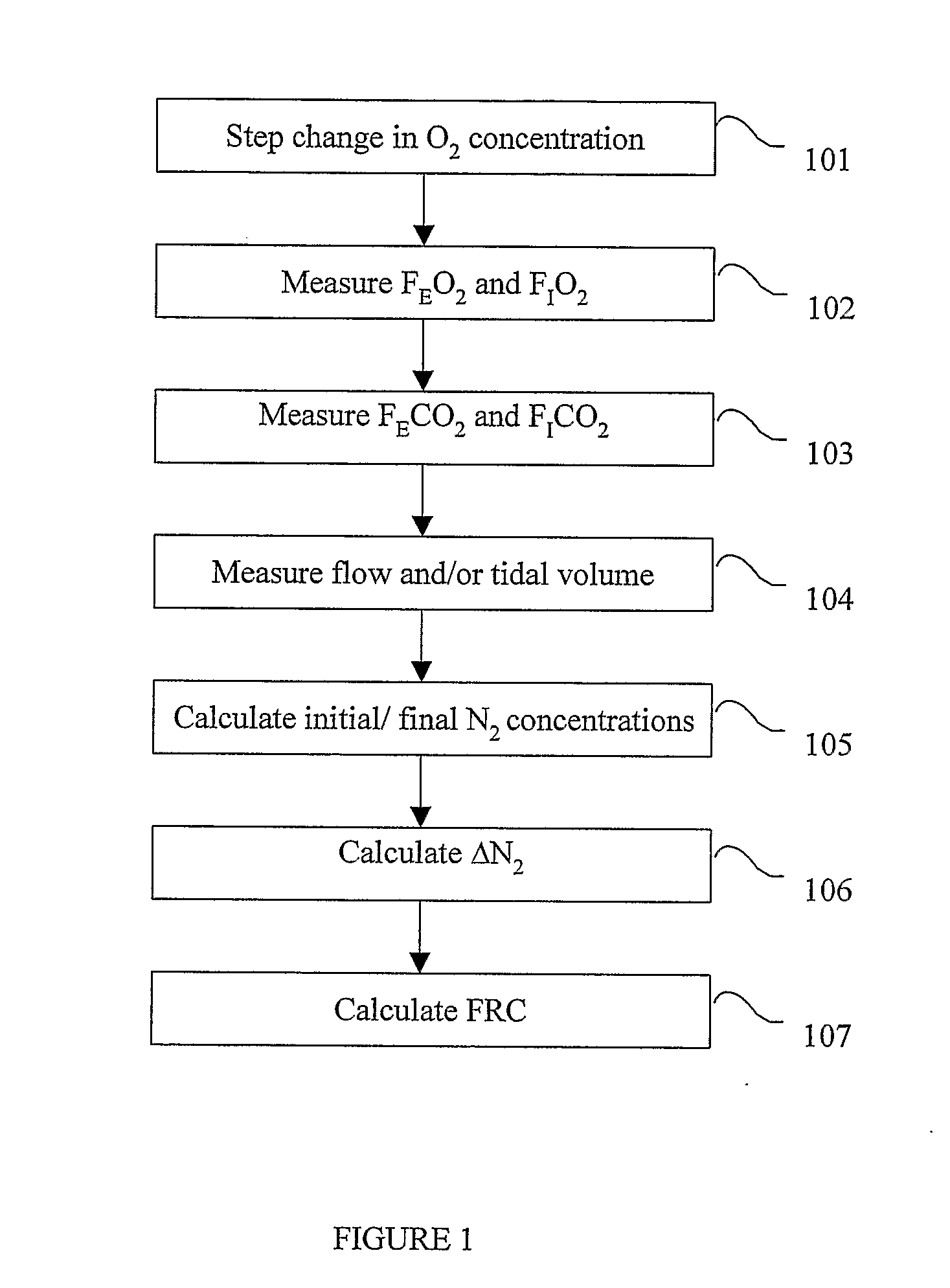
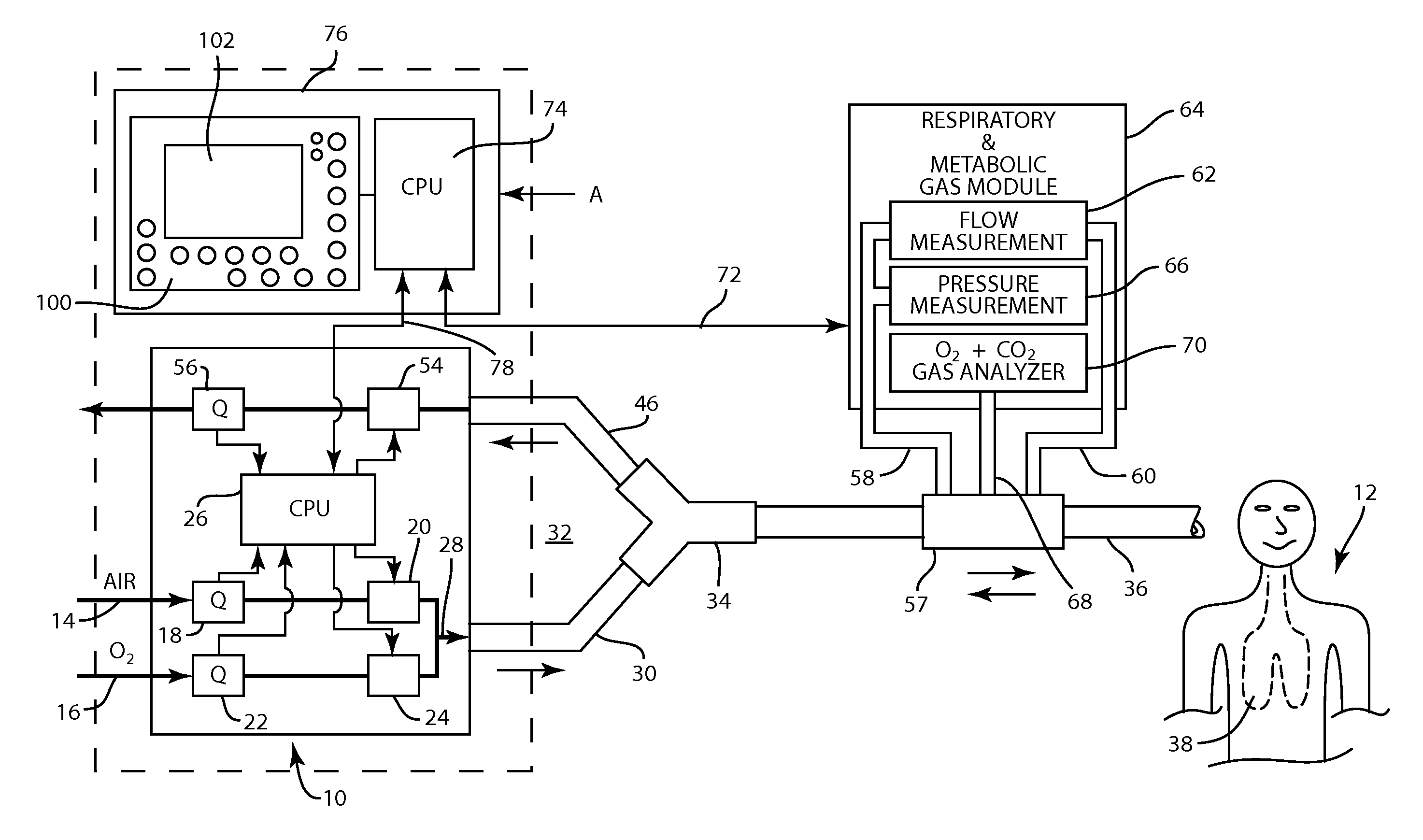
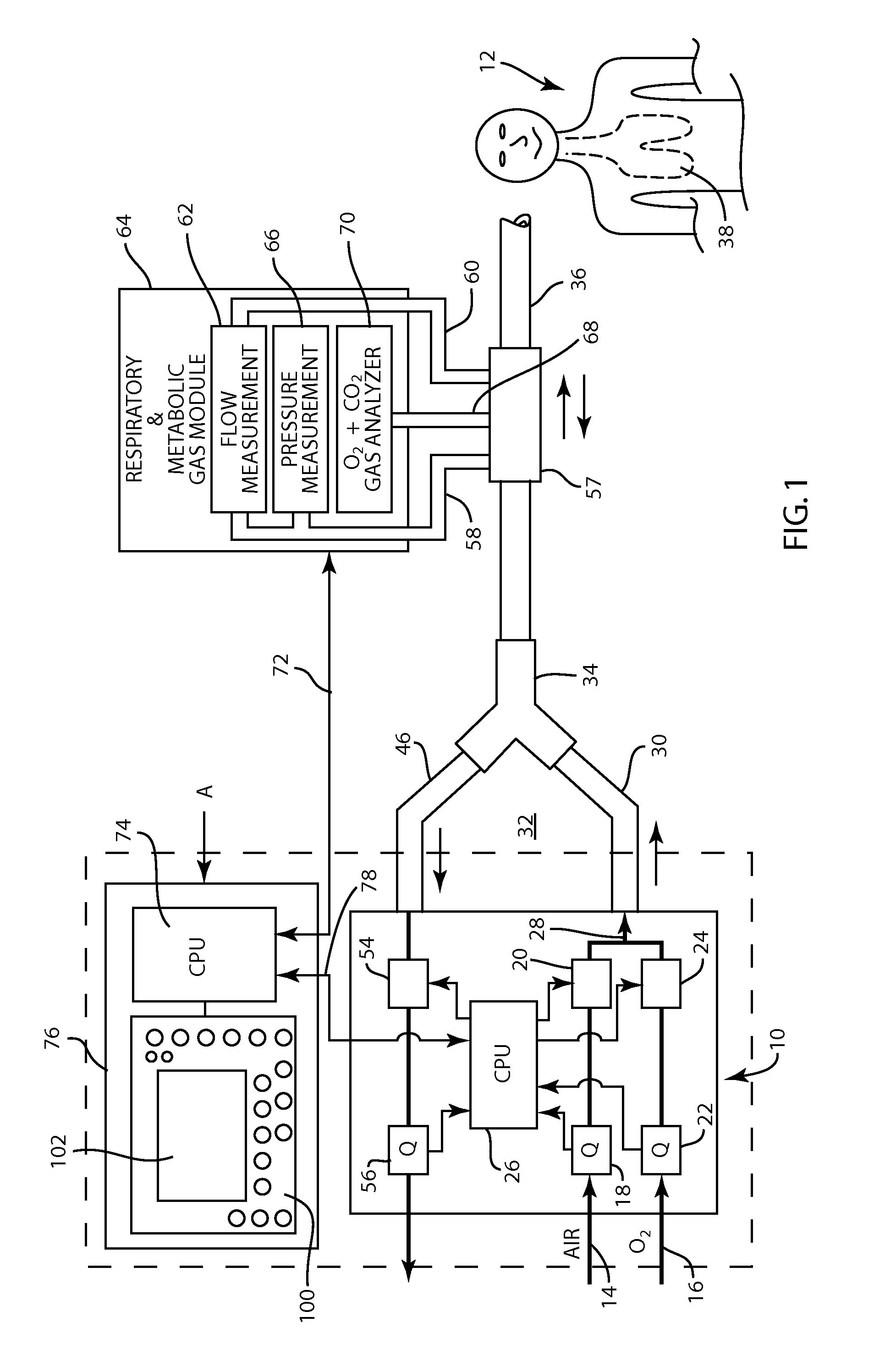
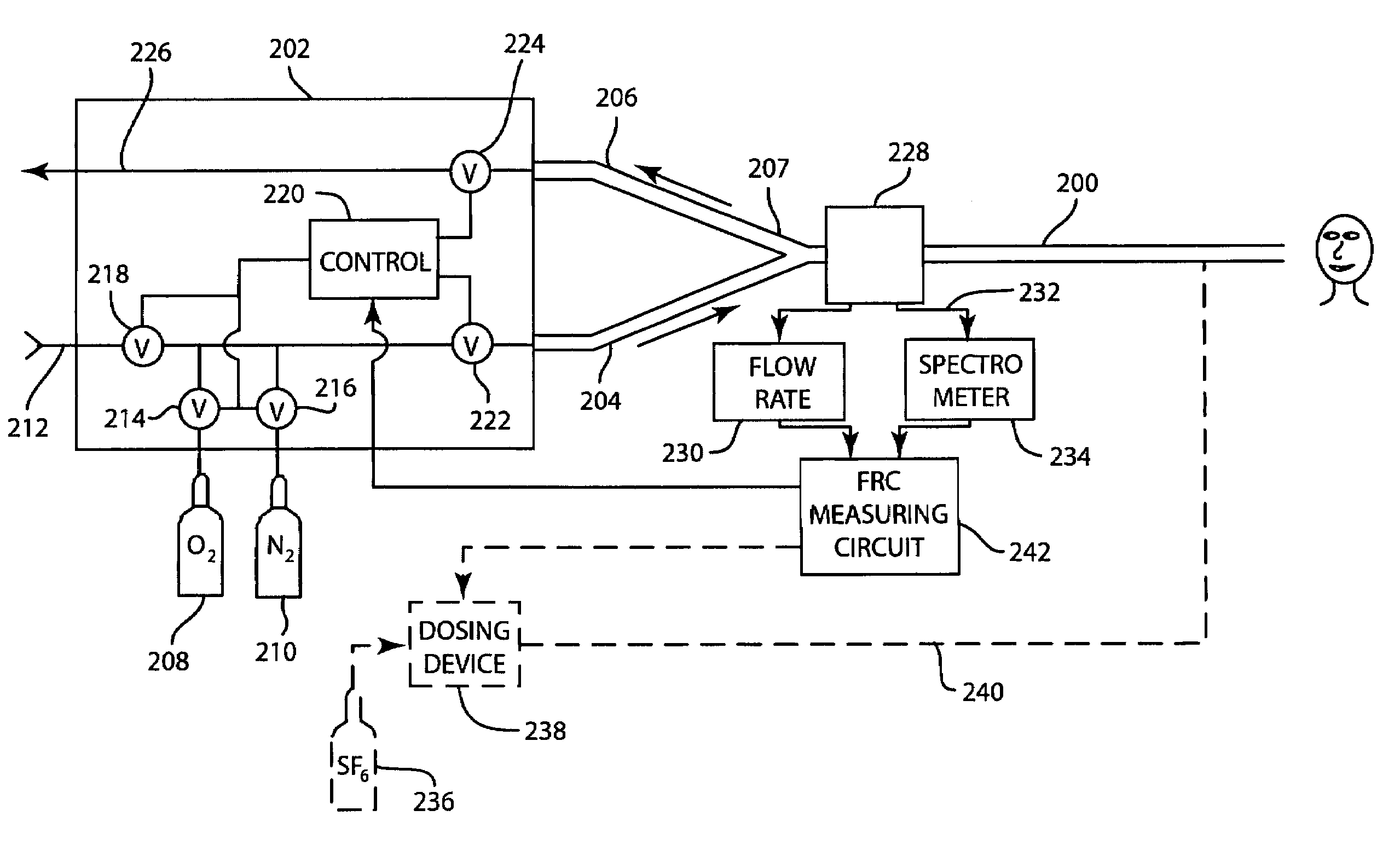
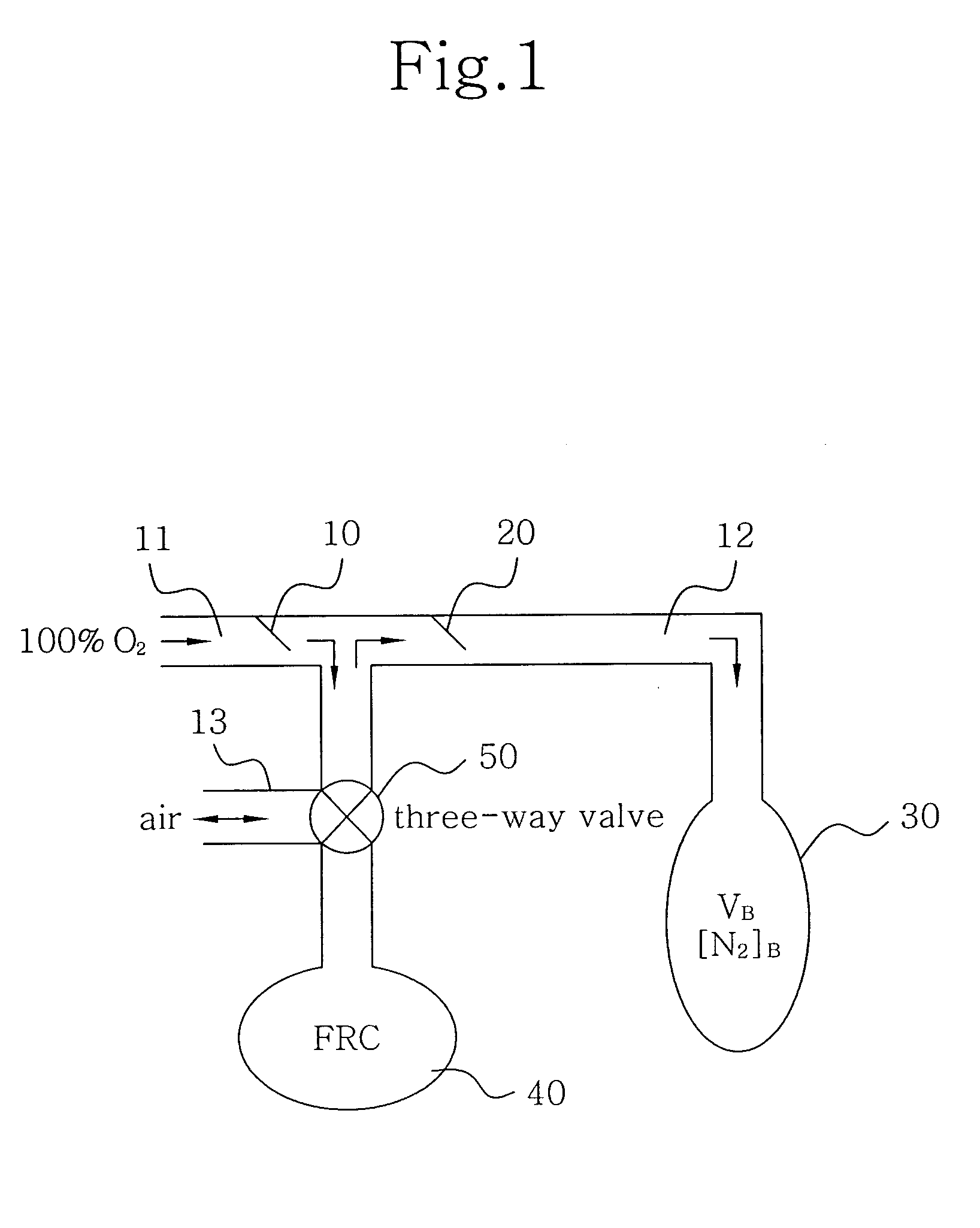
A method for determining functional residual capacity (FRC) of a patient while ventilating the patient. The system may include a medical ventilator which provides inhalation and exhalation from the patient, a sensor which measures a fraction one or more gas components of the inhalation and a fraction of the same gas components of the exhalation. A step change of oxygen fraction is provided to the inhalation of the patient. Subsequent to the step change, The fractions of the gas components are measured in the inhalation and in the exhalation. The functional residual capacity of the lungs of the patient is measured based on the fraction of the gas components in the inhalation and in the exhalation. The step change is provided manually by a technician, or automatically by programming a programmable device to provide the step change automatically to the patient by the medical ventilator. The step change is either an increase or a decrease in of the oxygen fraction.
Owner:TECHNION CITY
Noninvasive effective lung volume estimation
InactiveUS7699788B2Minimal invasivenessWithdrawing sample devicesRespiratory organ evaluationEstimation methodsIntensive care medicine
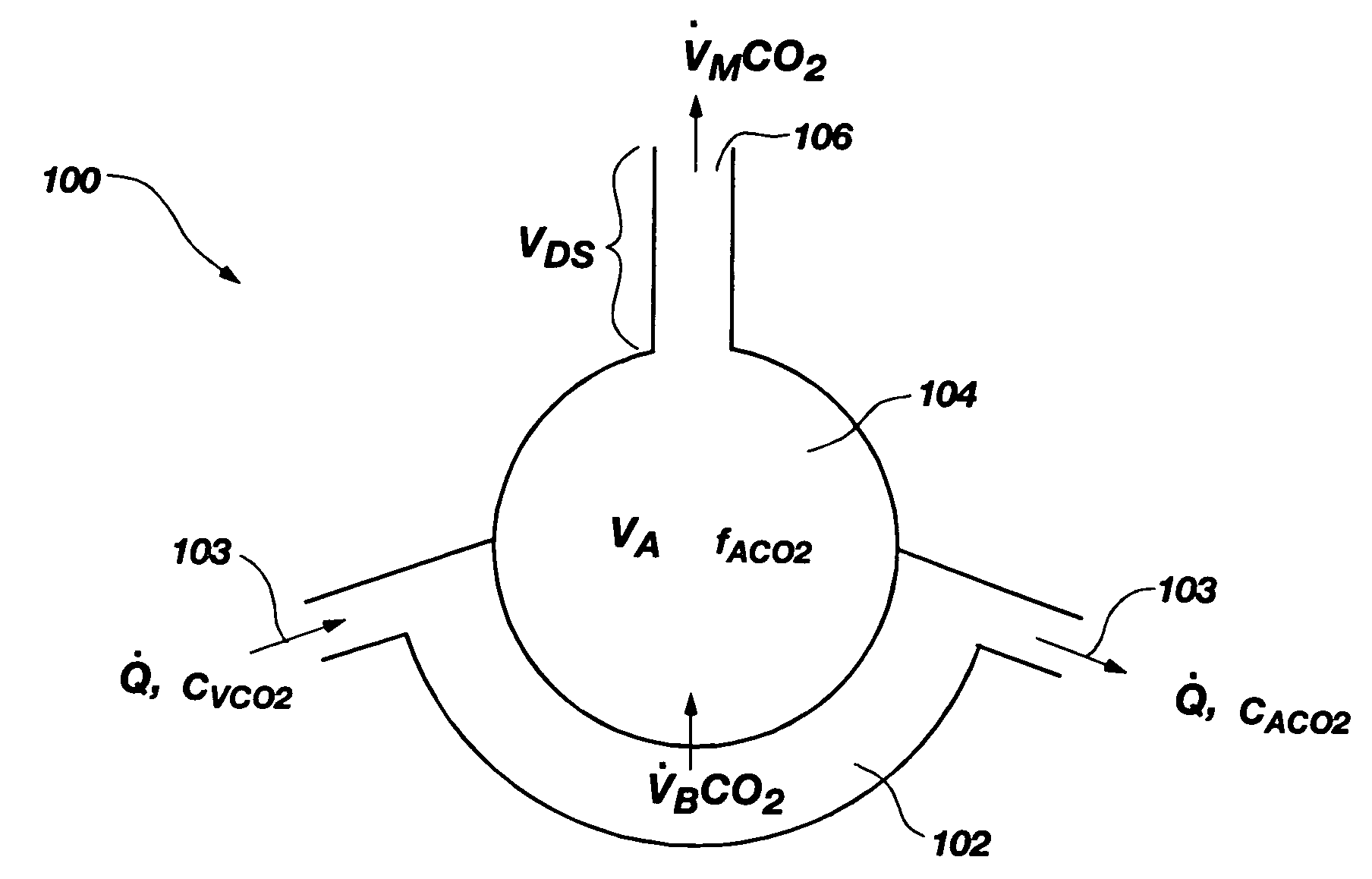
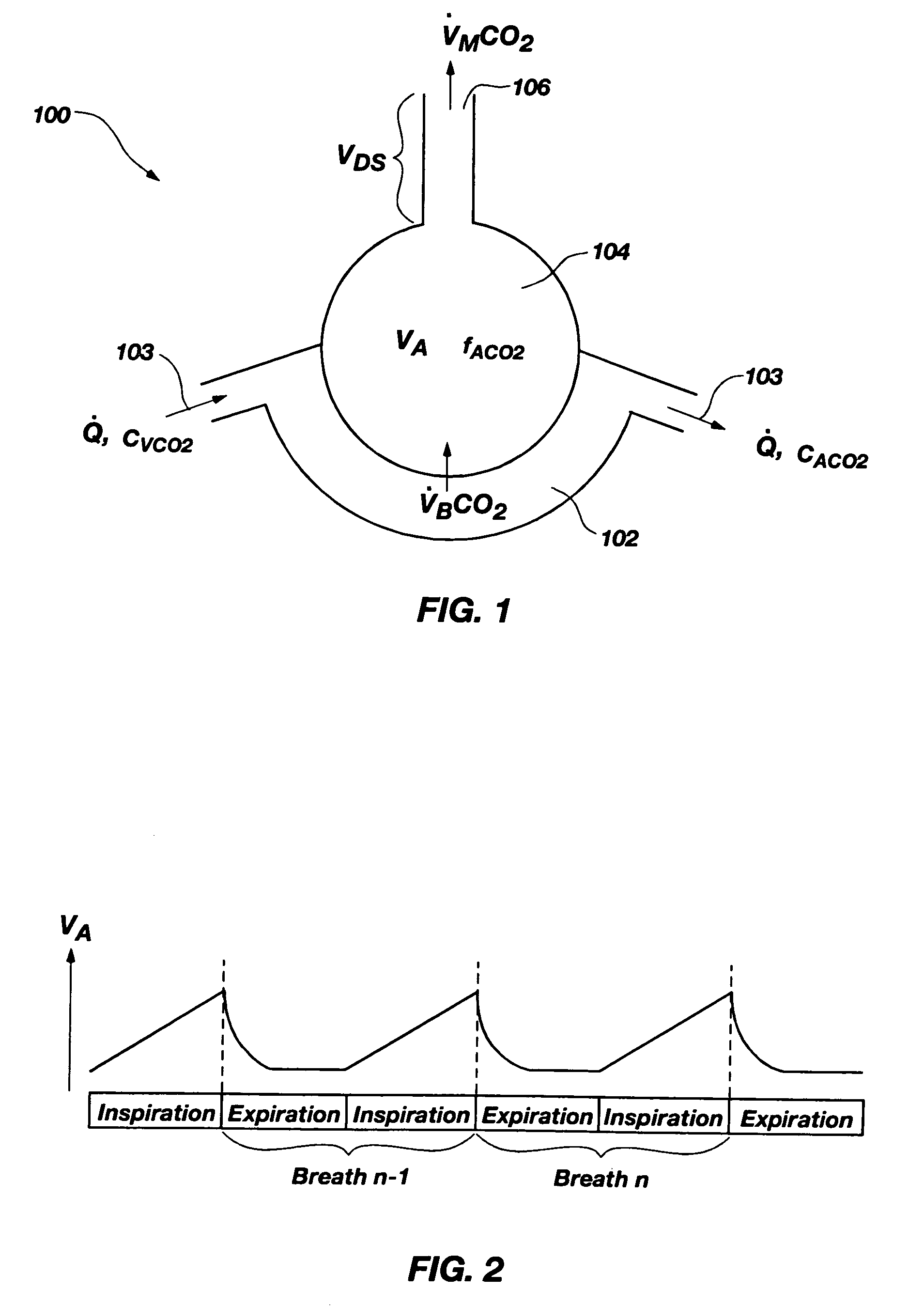
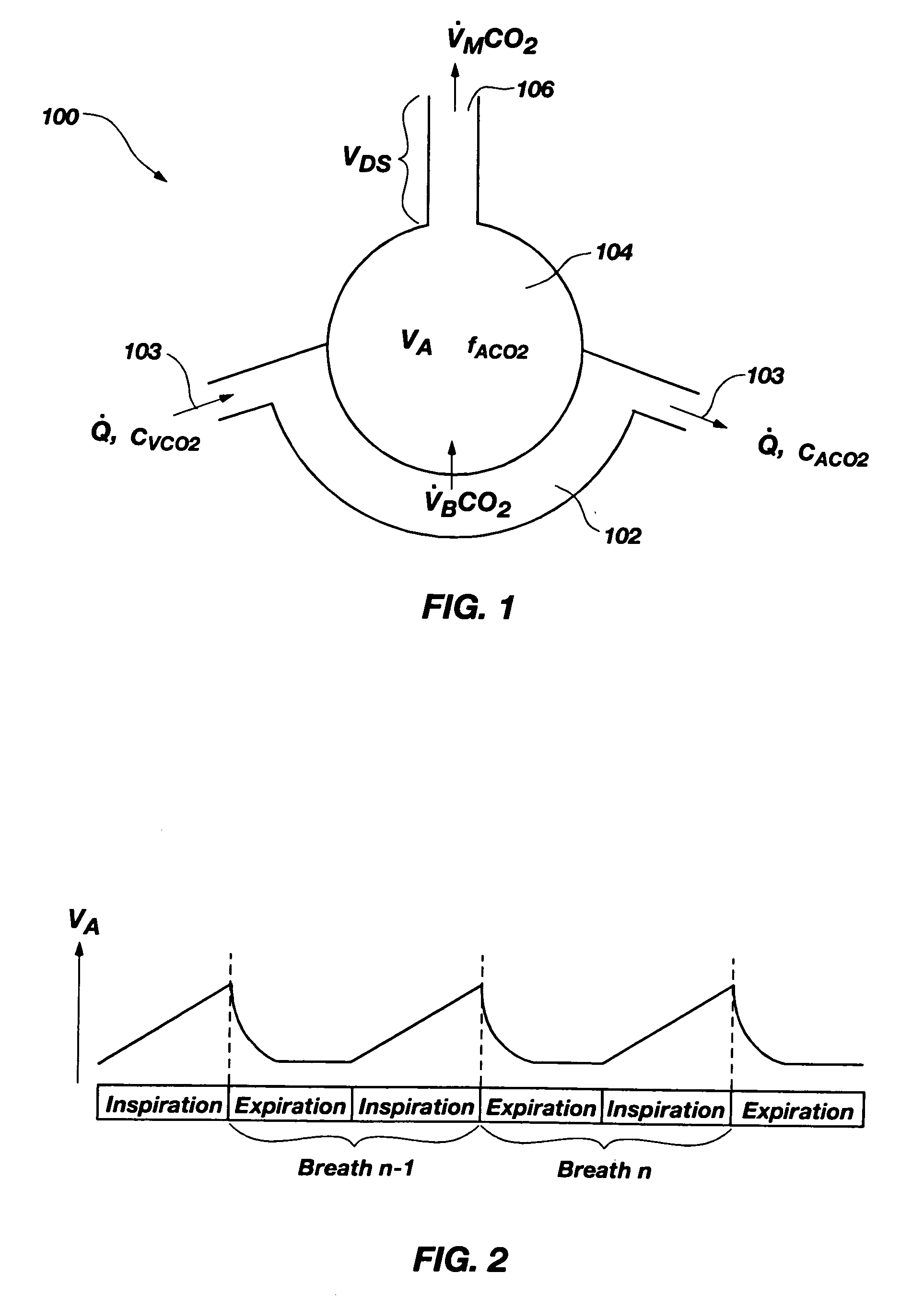
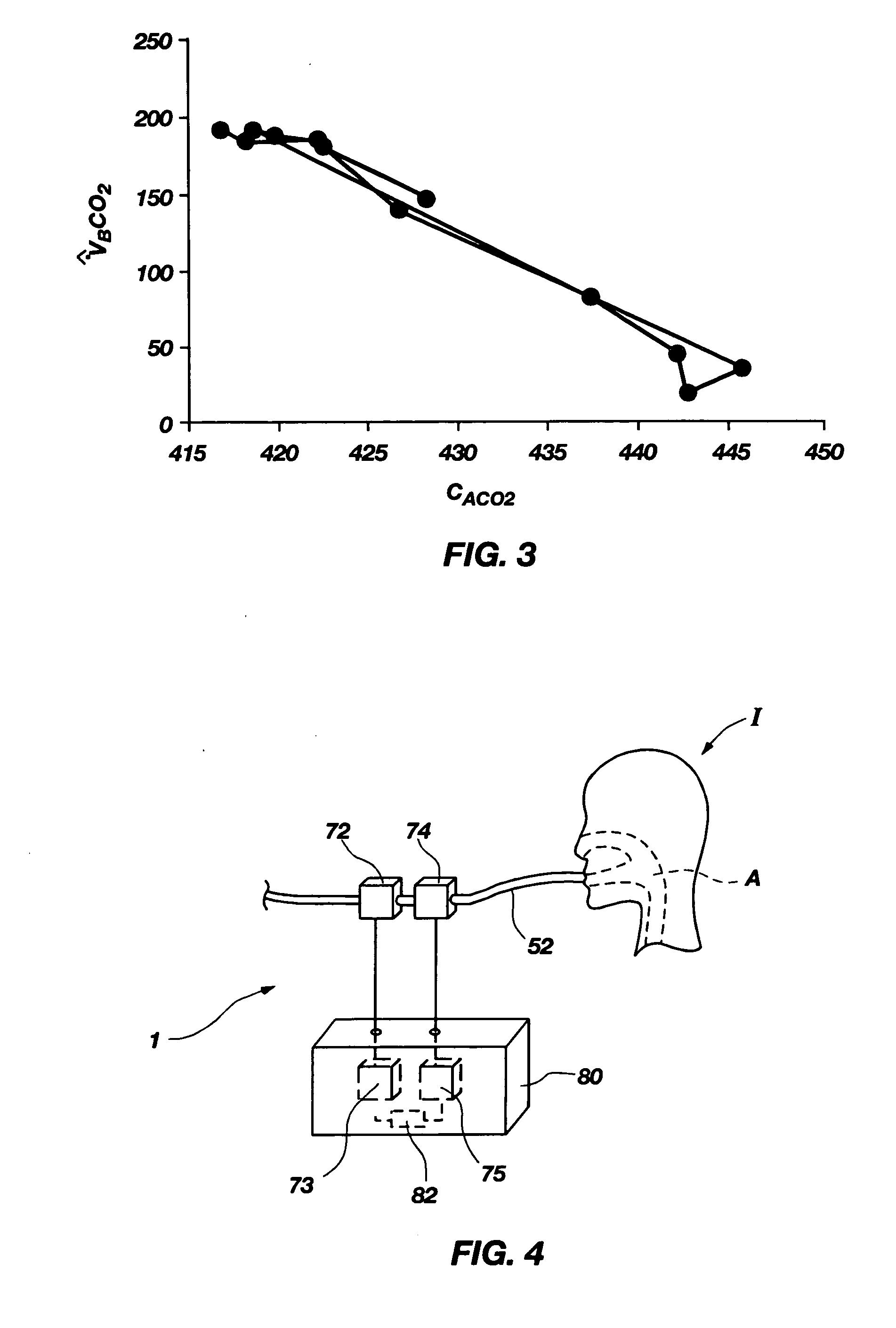
Methods for noninvasively measuring, or estimating, functional residual capacity or effective lung volume include obtaining carbon dioxide and flow measurements at or near the mouth of a subject. Such measurements are obtained during baseline breathing and during and shortly after inducement of a change in the subject's effective ventilation. The obtained measurements are evaluated to determine the amount of time required for exhaled carbon dioxide levels to return to normal—effectively an evaluation of carbon dioxide “washout” from the subject's lungs. Conversely, carbon dioxide and flow measurements may be evaluated to determine the amount of time it takes carbon dioxide to “wash in,” or reach peak levels within, the lungs of the subject following the change in the subject's effective ventilation. Apparatus for effective such methods are also disclosed.
Owner:RIC INVESTMENTS LLC
Method and apparatus for breathing during anesthesia
InactiveUS6123072AEliminate the problemMinimize movementRespiratorsOperating means/releasing devices for valvesAutonomous breathingWhole body
A method and apparatus are provided for ventilation of patients during general anesthesia. Breathing gas is supplied to the patient during anesthesia at a controlled volume above the functional residual capacity of the patient's lungs. The patient is allowed to spontaneously respire when the volume of breathing gas is above the functional residual capacity. The pressure of the breathing gas is periodically reduced to facilitate expulsion of carbon dioxide-containing gas from the patient. The system promotes alveolar ventilation, carbon dioxide excretion, oxygenation and respiratory monitoring in patients who receive general anesthesia.
Owner:DOWNS JOHN B
Device and process for controlling a respirator
ActiveUS20060260611A1Local changeImprove ventilationRespiratorsOperating means/releasing devices for valvesFRC - Functional residual capacityRespirator
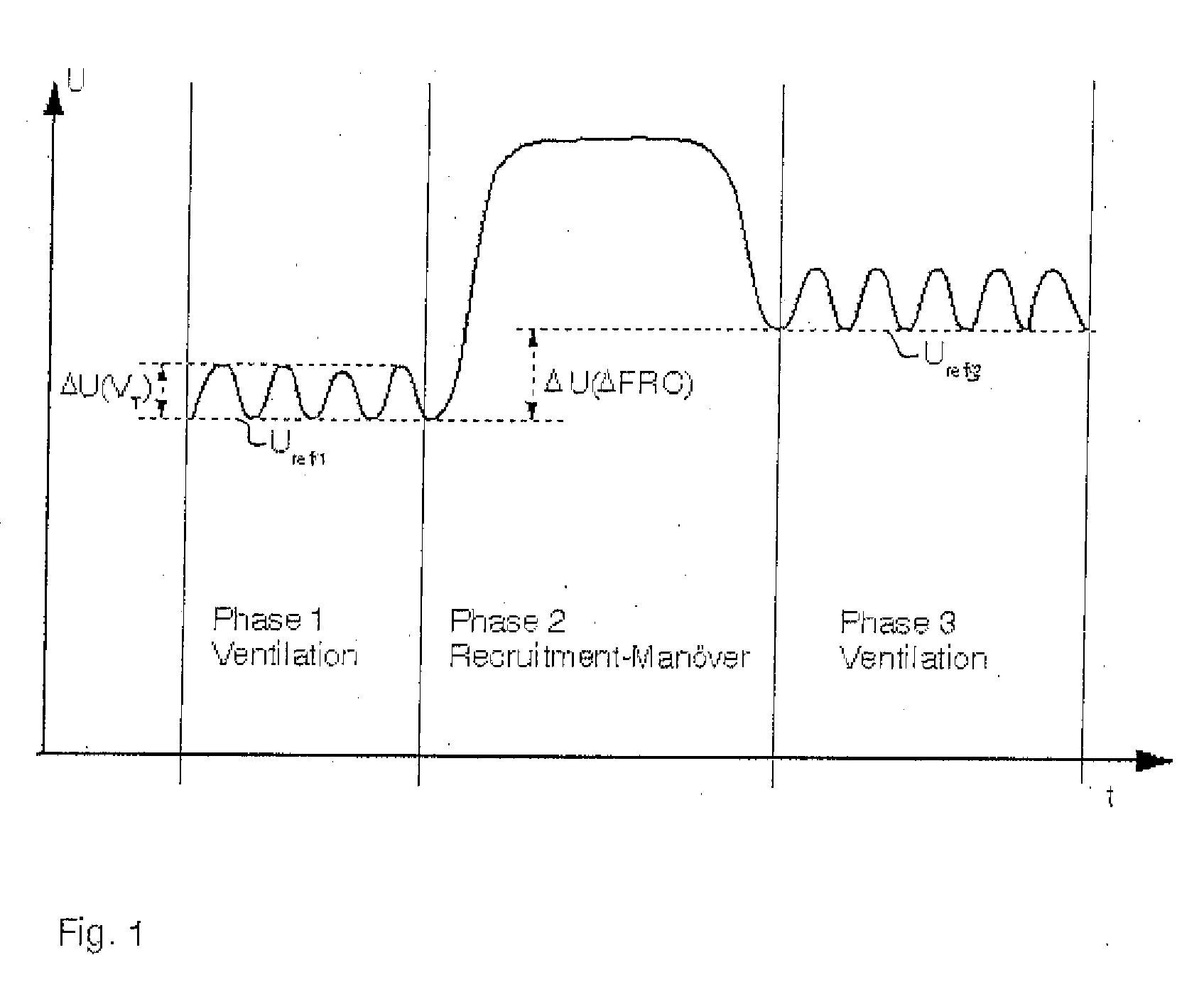
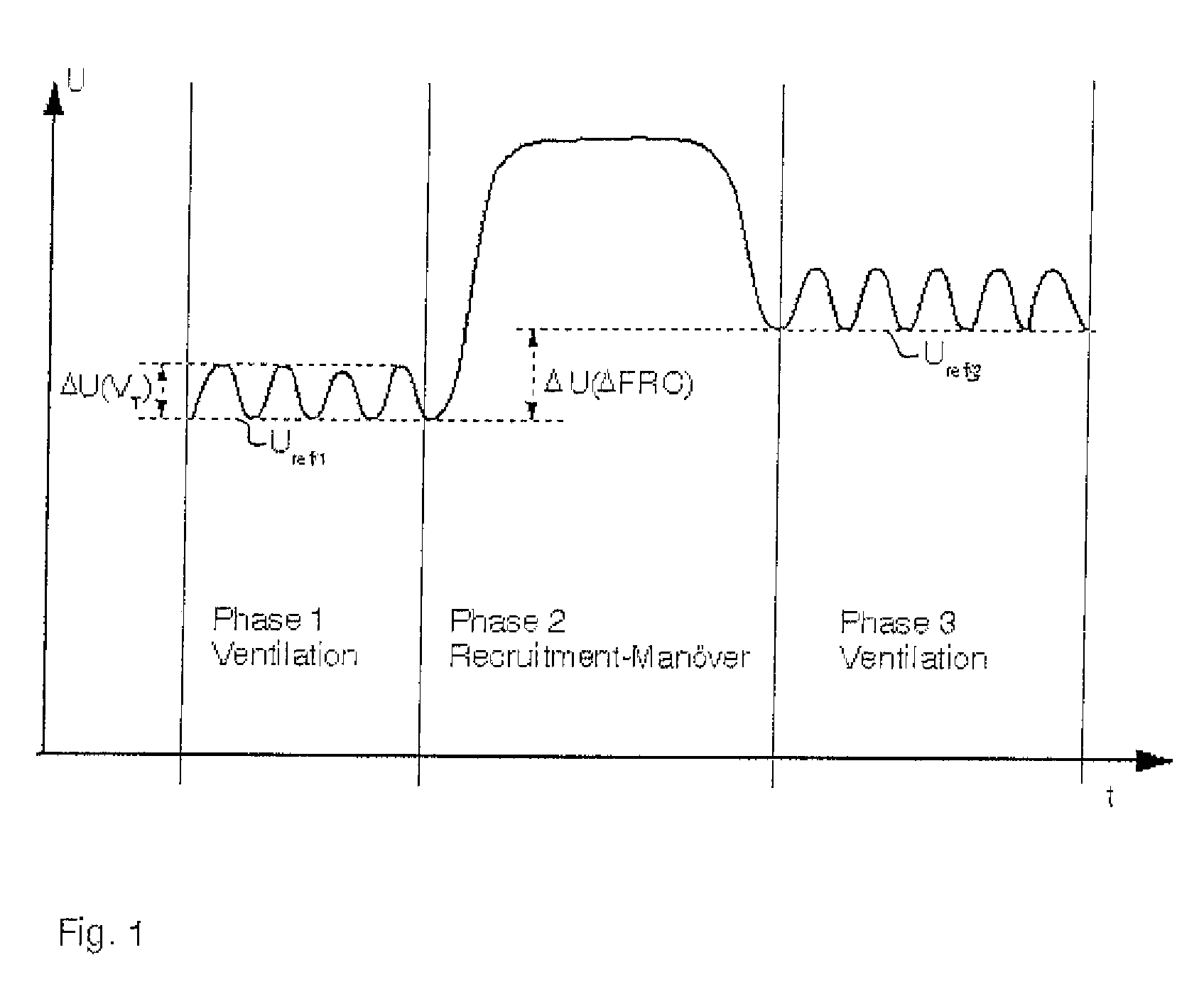
A device and a process for determining the change in the functional residual capacity (FRC) in a simple manner. Based on a first respiration phase for mechanical respiration, a recruitment maneuver is performed for this during a second respiration phase, and respiration is switched back to mechanical respiration during a third respiration phase. Reference values Uref / 1, Uref / 3 are formed from the end-expiratory values of the impedance measured signals U during the first respiration phase and the third respiration phase, and the difference ΔU (ΔFRC) between the reference value Uref / 3 of the third respiration phase and the reference value Uref / 1 of the first respiration phase is an indicator of the change in the functional residual capacity of the lung of the test subject.
Owner:DRAGERWERK AG
Apparatus for calculating residual capacity of secondary battery
ActiveUS20120166116A1Accurate calculationWide voltage rangeElectrical testingSpecial data processing applicationsElectrical batteryEngineering
An apparatus for calculating a residual capacity of a secondary battery is provided. The apparatus calculates the residual capacity of energy in the secondary battery which is charged / discharged. The apparatus includes an arithmetic unit which estimates and calculates a first residual capacity based on a charge / discharge voltage corresponding to a residual capacity of the secondary battery, calculates a second residual capacity based on an integrated value of a charge / discharge current of the secondary battery, weights the charge / discharge voltage of the secondary battery with the first residual capacity or the second residual capacity according to the voltage changing rate, and combines the results of the weighting to obtain the residual capacity of the secondary battery.
Owner:DENSO CORP
Method and apparatus for measuring functional residual capacity (FRC) and cardiac output(CO)
InactiveUS7465275B2Easy to measureEasy to calculateWithdrawing sample devicesCatheterExcretion processOxygen
The present invention relates to a method and an apparatus for measuring Functional Residual Capacity (CO). Further, it relates to a method and an apparatus for measuring cardiac output (CO). The method comprises the steps of determining the amount of oxygen and carbon dioxide being inhaled and exhaled during at least two breathing cycles, identifying at least one difference between said breathing cycles, and estimating the FRC and / or CO based on the determined uptake of oxygen and excretion of carbon dioxide and said identified difference.
Owner:GE HEALTHCARE FINLAND
Device and method for manipulating exhalation
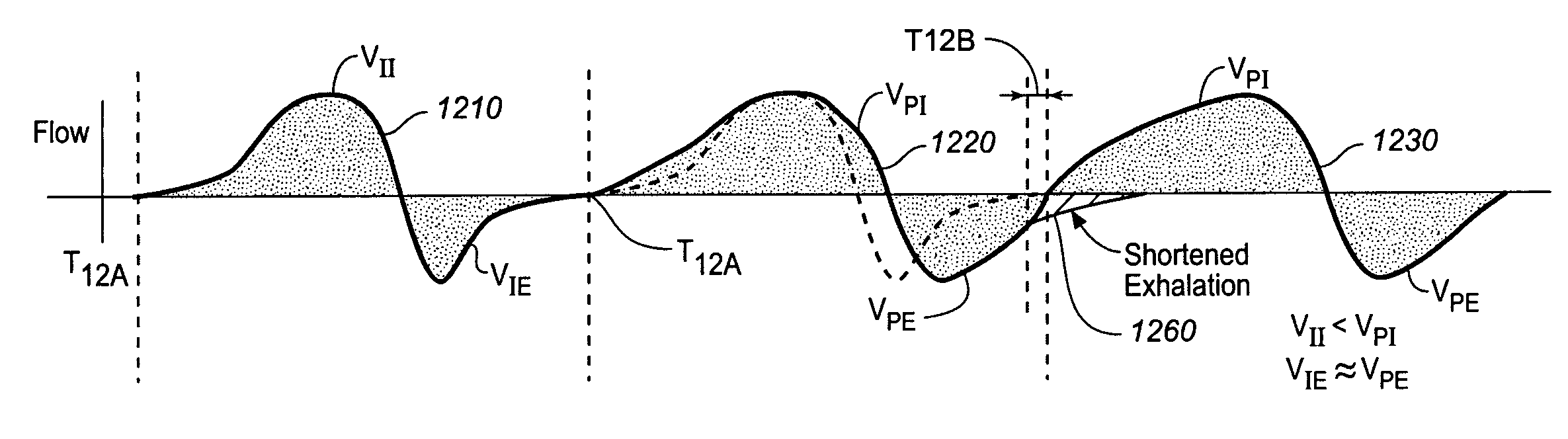
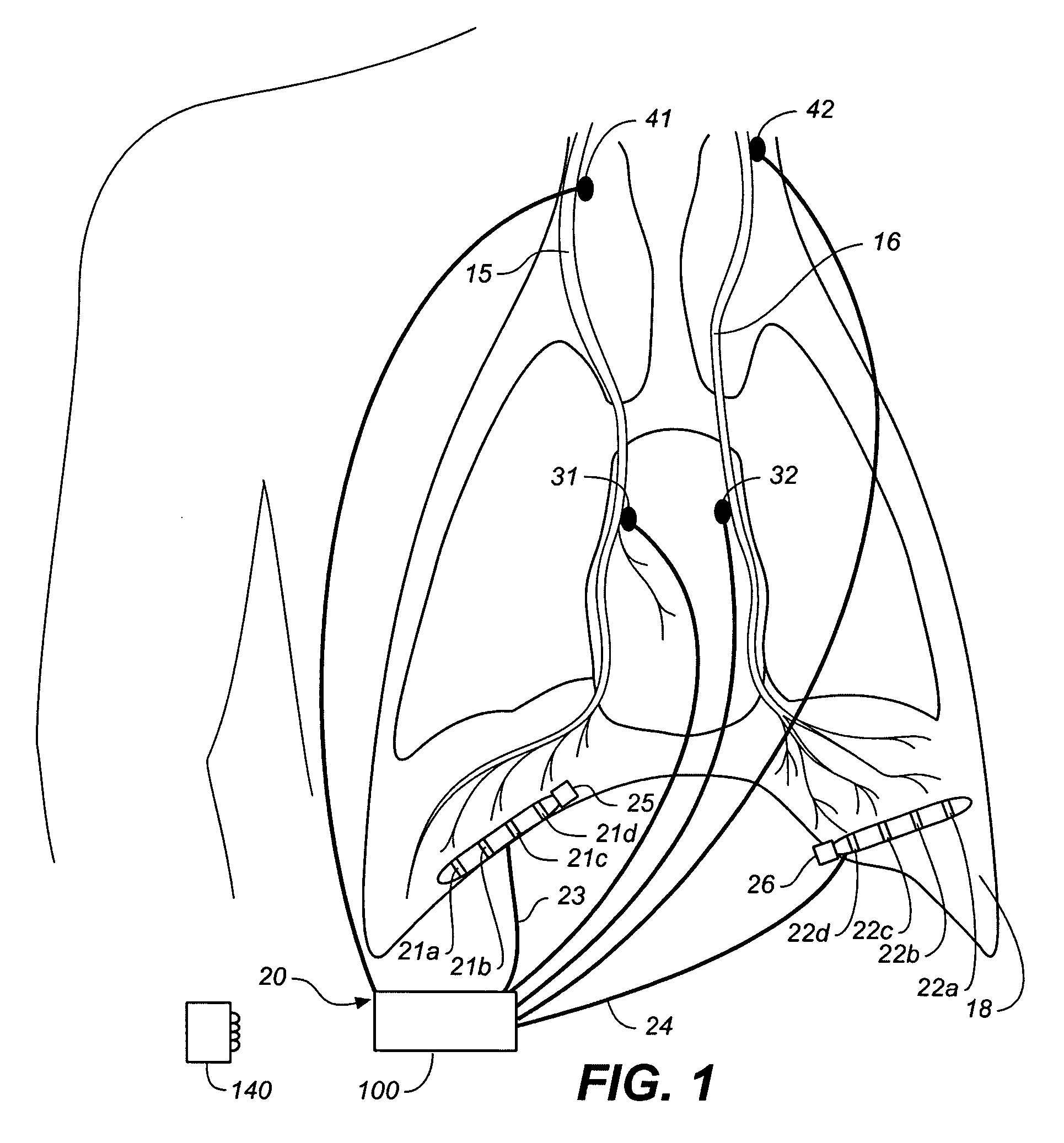
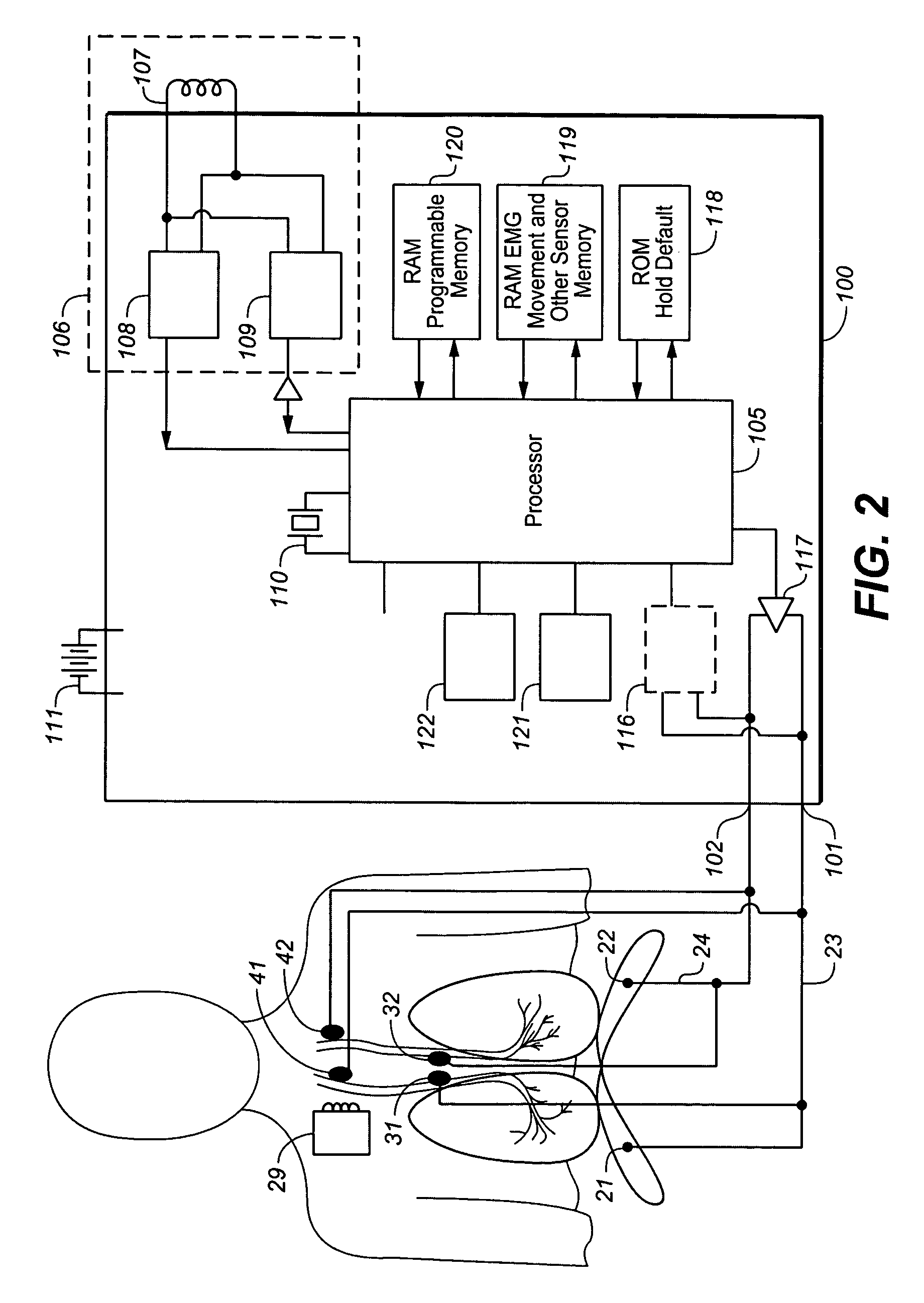
ActiveUS9259573B2Prevent obstructive respiratory eventsReduce generationElectrotherapyElectromyographyRadiologyFunctional residual capacity
A device and method is provided for increasing the functional residual capacity of lungs of a subject by electrically stimulating tissue associated with the diaphragm or phrenic nerve of the subject.
Owner:RMX
Noninvasive effective lung volume estimation
InactiveUS20050124907A1Minimal invasivenessLayered productsWithdrawing sample devicesEstimation methodsWashout
Methods for noninvasively measuring, or estimating, functional residual capacity or effective lung volume include obtaining carbon dioxide and flow measurements at or near the mouth of a subject. Such measurements are obtained during baseline breathing and during and shortly after inducement of a change in the subject's effective ventilation. The obtained measurements are evaluated to determine the amount of time required for exhaled carbon dioxide levels to return to normal—effectively an evaluation of carbon dioxide “washout” from the subject's lungs. Conversely, carbon dioxide and flow measurements may be evaluated to determine the amount of time it takes carbon dioxide to “wash in,” or reach peak levels within, the lungs of the subject following the change in the subject's effective ventilation. Apparatus for effective such methods are also disclosed.
Owner:RIC INVESTMENTS LLC
Device and process for controlling a respirator
ActiveUS7584752B2Improve ventilationRespiratorsOperating means/releasing devices for valvesFRC - Functional residual capacityRespirator
A device and a process for determining the change in the functional residual capacity (FRC) in a simple manner. Based on a first respiration phase for mechanical respiration, a recruitment maneuver is performed for this during a second respiration phase, and respiration is switched back to mechanical respiration during a third respiration phase. Reference values Uref / 1, Uref / 3 are formed from the end-expiratory values of the impedance measured signals U during the first respiration phase and the third respiration phase, and the difference ΔU (ΔFRC) between the reference value Uref / 3 of the third respiration phase and the reference value Uref / 1 of the first respiration phase is an indicator of the change in the functional residual capacity of the lung of the test subject.
Owner:DRAGERWERK AG
Plethysmograph
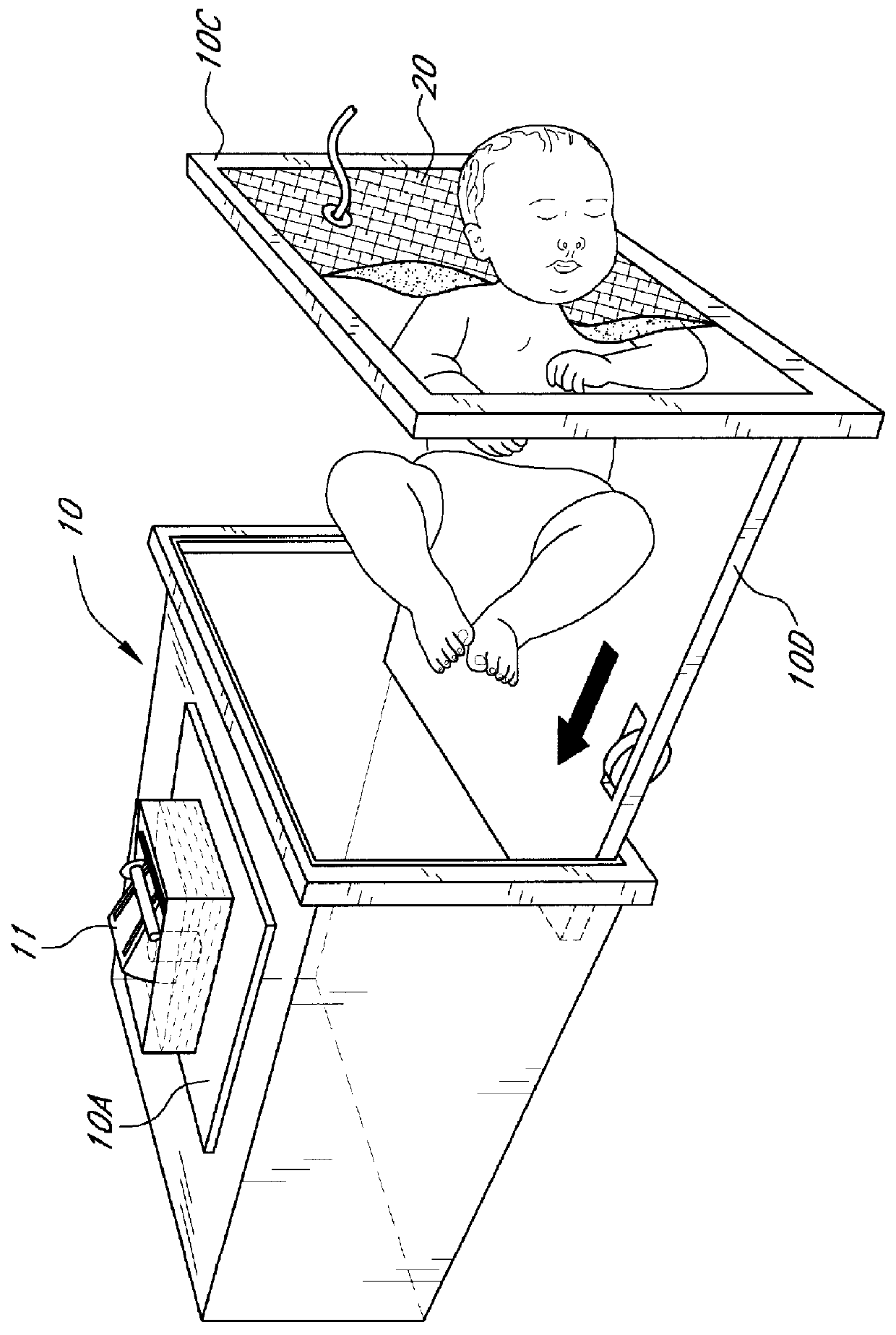
InactiveUS6113550ACheap constructionBreathe freelyBaby-incubatorsRespiratory organ evaluationTidal volumeNon invasive
Non invasive measurement of infant lung function during unsedated sleep is achieved by a plethysmograph which comprises of a rigid acrylic box (10) with integral water sealed spirometer (11) and a vacuum-actuated neck seal (20) allowing "head out" monitoring. The neck seal comprises particulate material entrapped in the space between two layers of rubber, which become rigid when a vacuum is applied to the space. Tidal volume, respiratory rate and changes in functional residual capacity (FRC) are able to be recorded during unsedated REM and NREM sleep whilst monitoring with conventional polysomnographic methods. The head out configuration allows additional instrumentation to be used, avoids facial stimulation and allows unimpeded access to the upper airway.
Owner:QUEENSLAND UNIV OF THE
Apparatus and Method for Identifying FRC and PEEP Characteristics
ActiveUS20120055476A1RespiratorsOperating means/releasing devices for valvesForced expiratory vital capacityPressure data
A patient ventilator for assisting a clinician in determining a suitable PEEP for the patient. The amount of the lung volume recruited / de-recruited at various levels of PEEP may be determined for use in selecting a desired PEEP. To this end, the functional residual capacity of the lungs is determined for a first PEEP level. The PEEP is then altered to a second level and a spirometry dynostatic curve of lung volume and pressure data is obtained. The lung volume on the dynostatic curve at a lung pressure corresponding to the first PEEP value is obtained. The difference between the functional residual capacity of the lungs at the first PEEP level and that determined from the dynostatic curve represents the lung volume recruited / de-recruited when changing between said first and second PEEPs.
Owner:GENERAL ELECTRIC CO
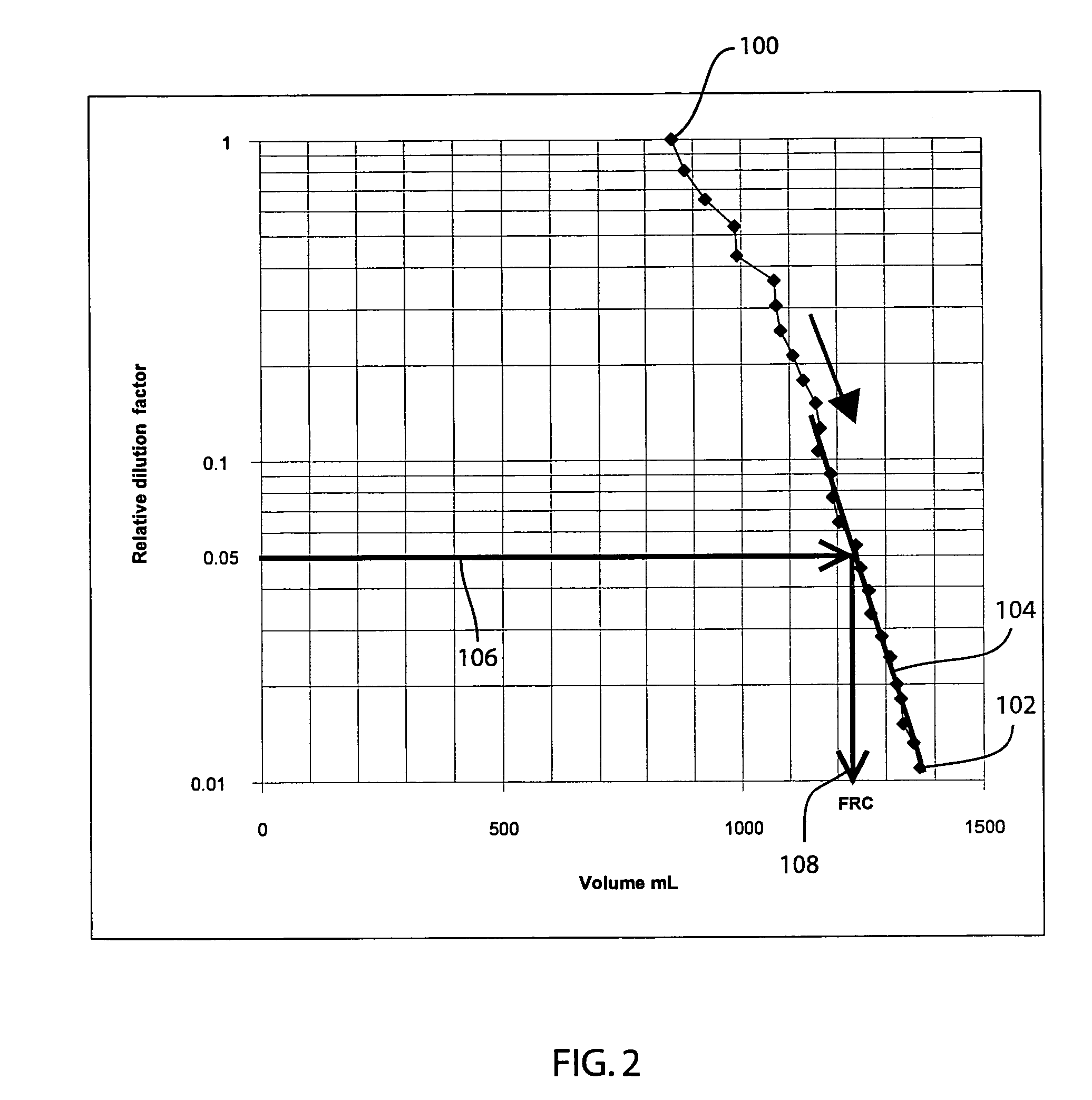
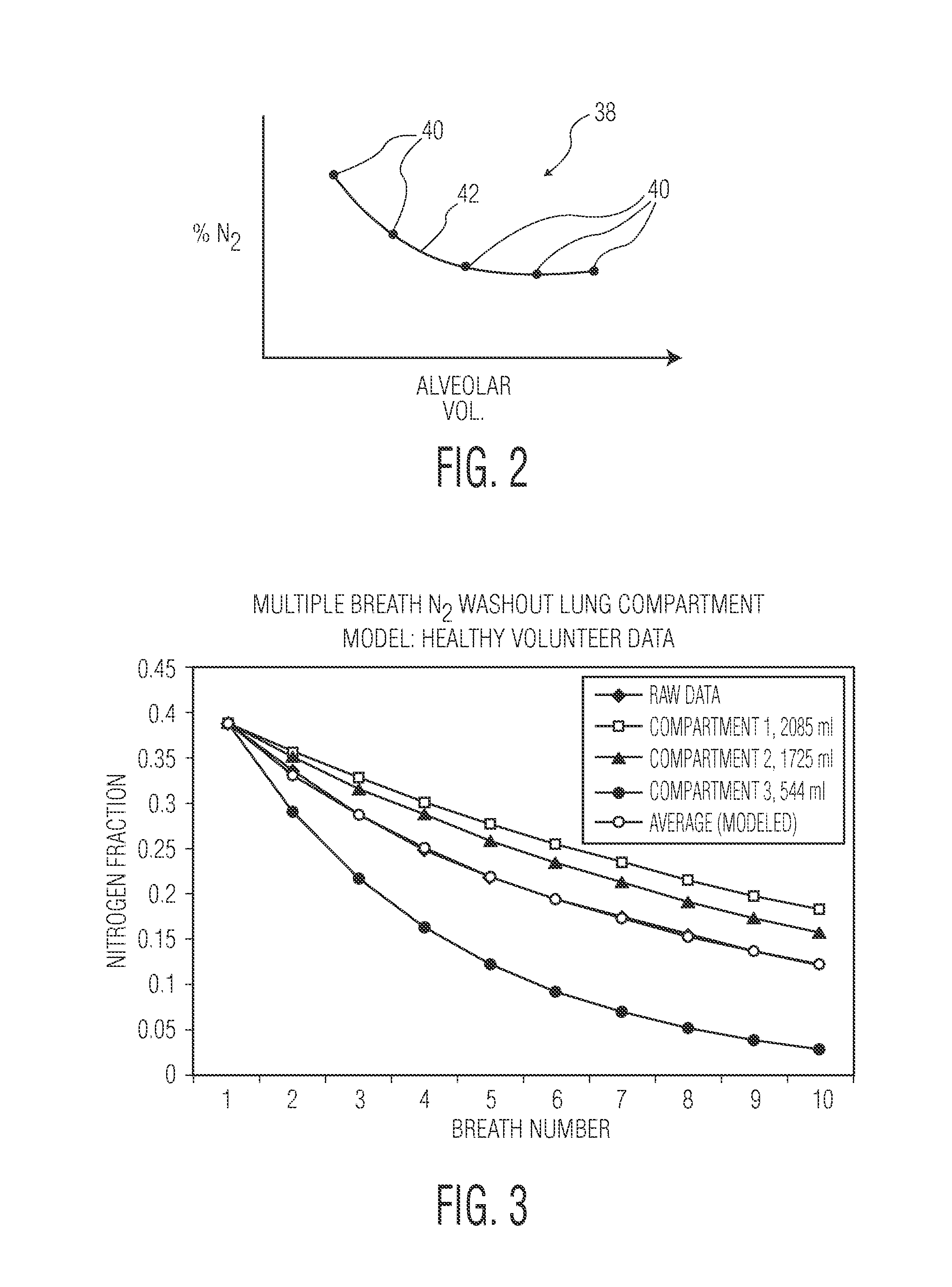
Method and apparatus for determining functional residual capacity of the lungs
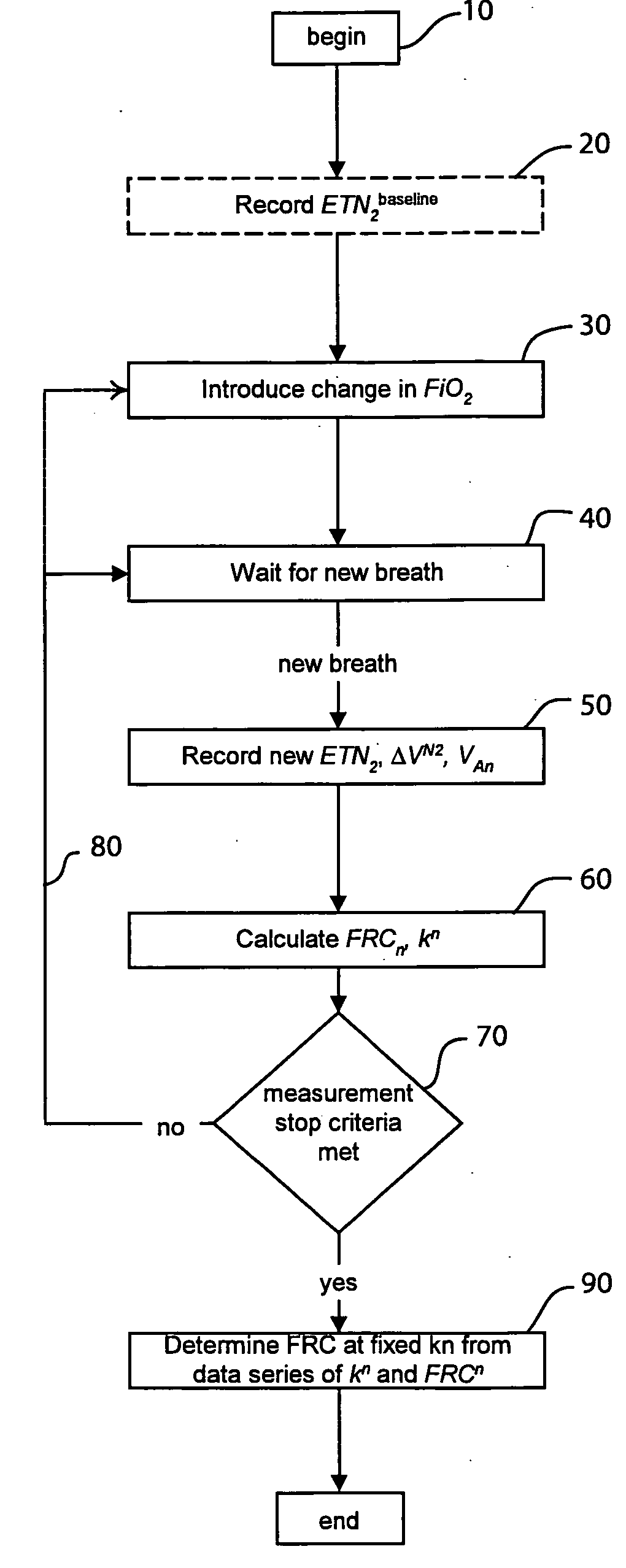
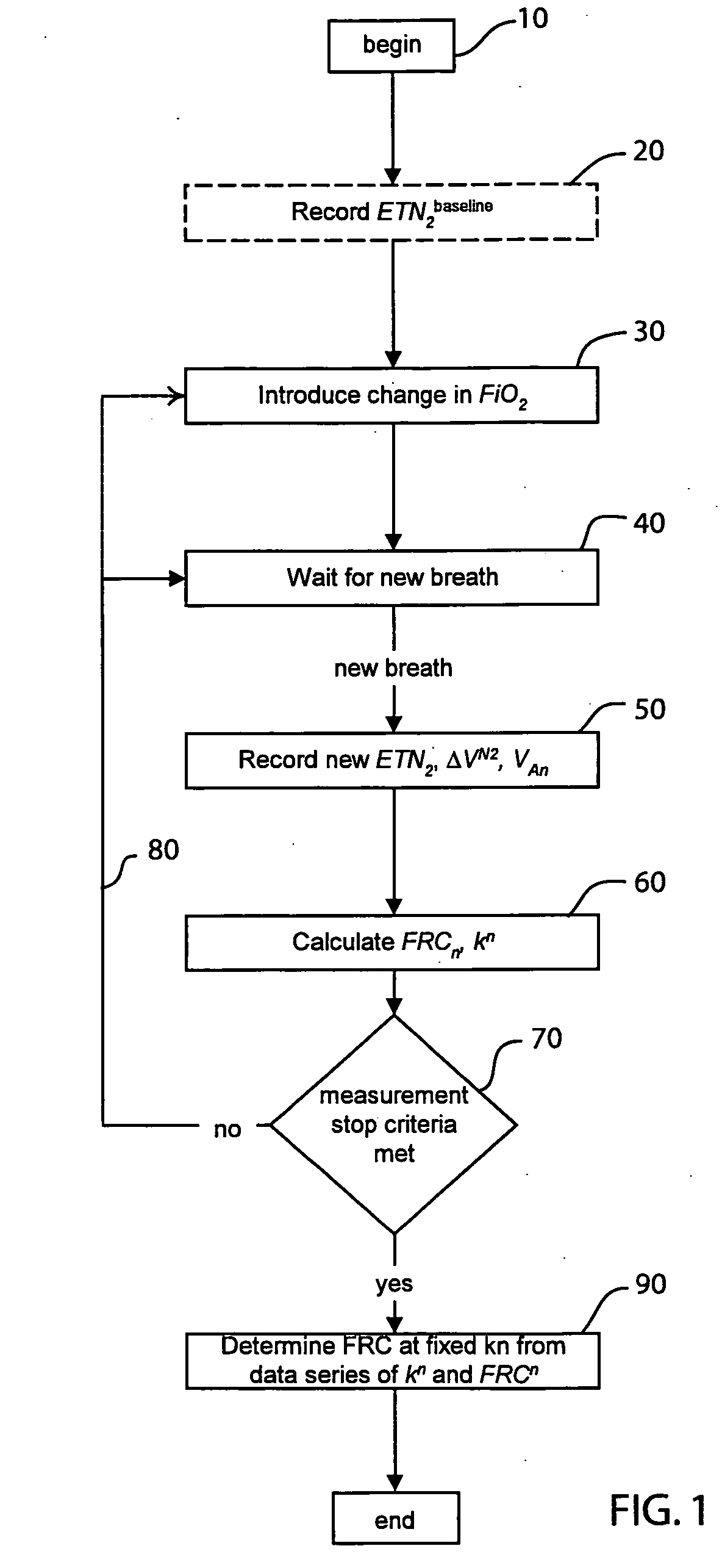
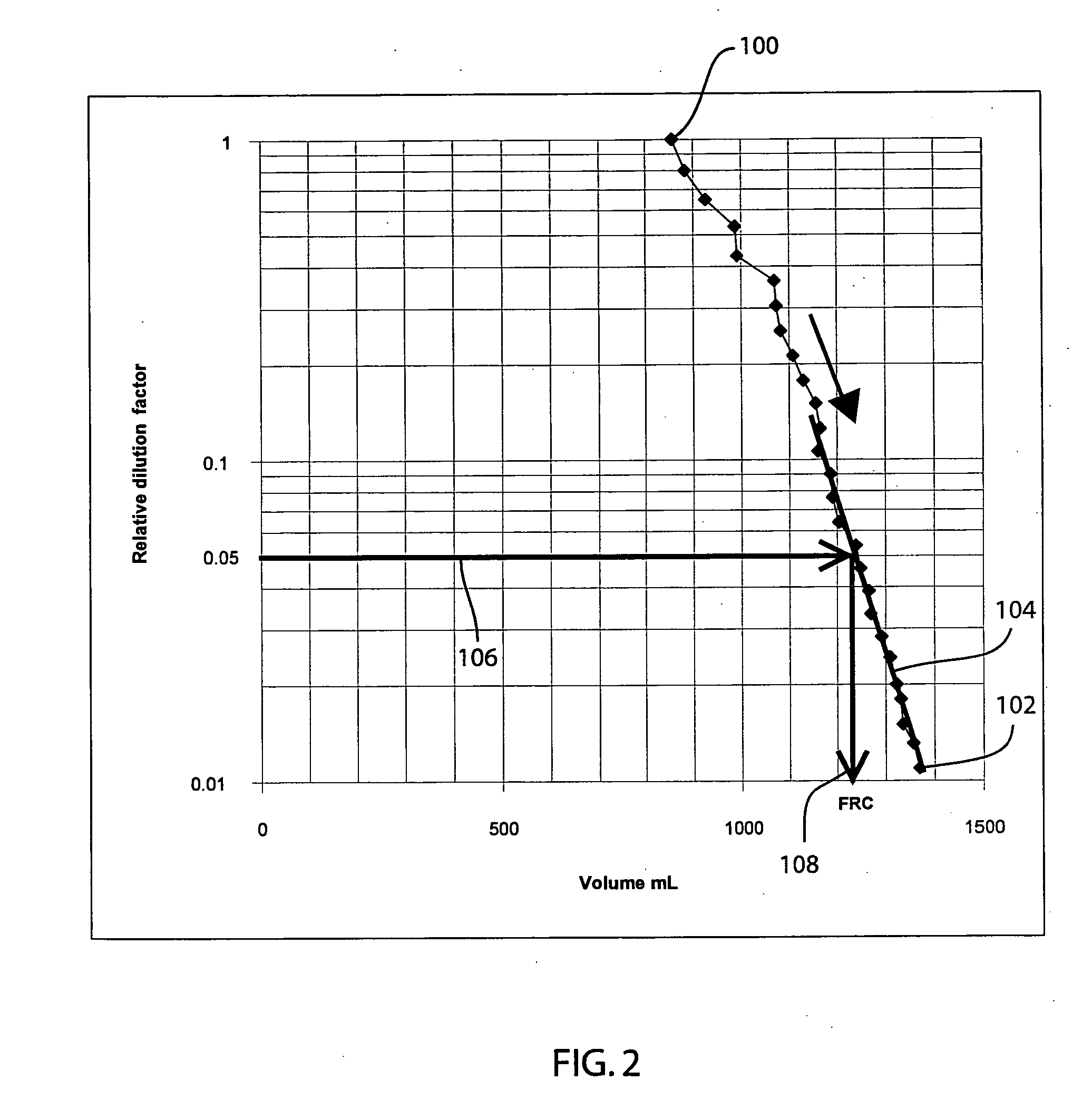
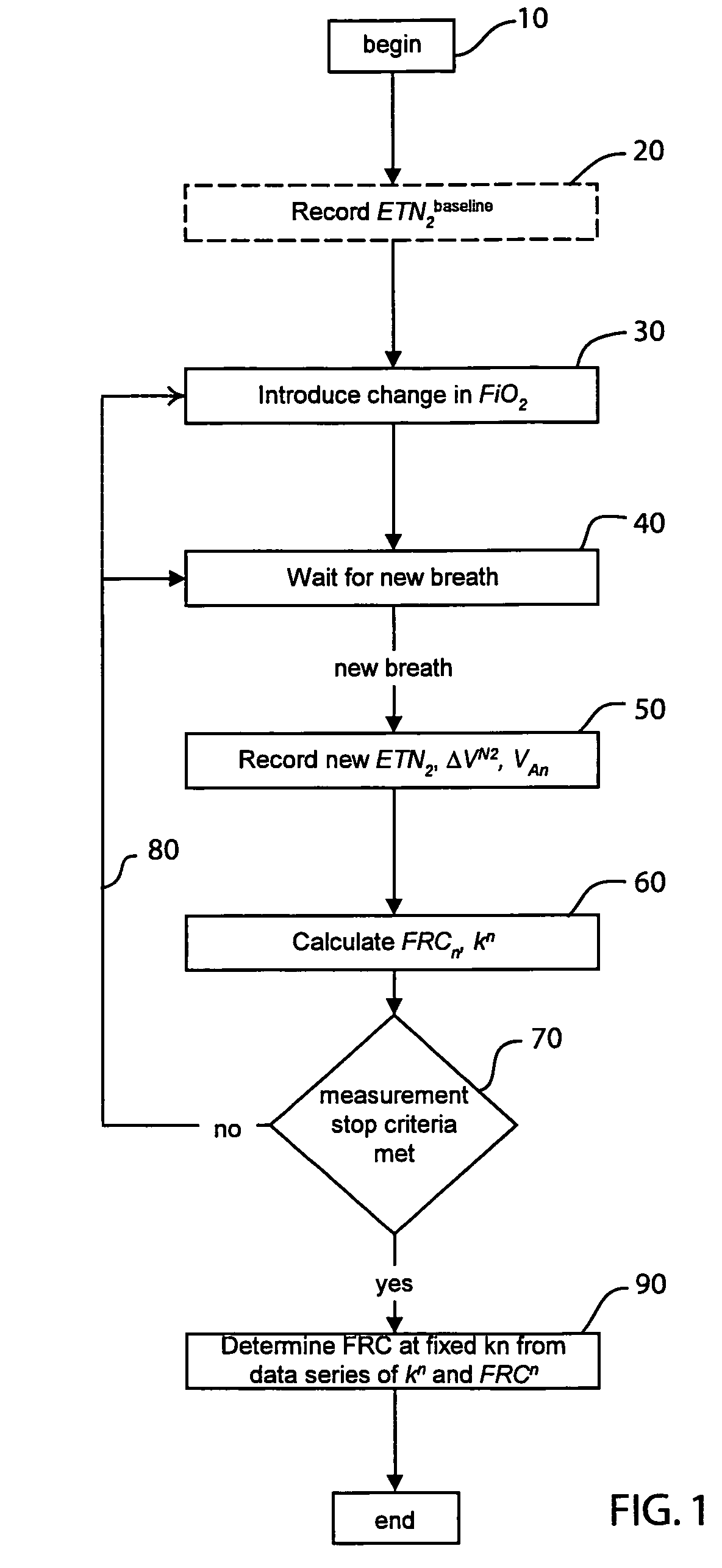
The functional residual capacity (FRC) value of a subject's lungs determined for each breath is related a volume dilution factor kn and the final FRC value is determined at the point where the volume dilution factor kn reaches a predetermined volume dilution factor value. The determination of the final FRC value can be based on interpolation or extrapolation from a measured breath data series. The volume dilution factor may be normalized value. This is achieved by division of all volume dilution factors with the initially determined volume dilution factor. With normalization, the compartments in which gas concentration change is achieved slowly are referenced with the faster, more effective compartments, thus giving a ventilation profile of the lungs.
Owner:GENERAL ELECTRIC CO
Equilibration method for high resolution imaging of lung compliance and distribution of functional residual capacity
InactiveUS7072706B2Fast imagingHigh resolutionRespiratory organ evaluationSensorsPresent methodHigh resolution imaging
The present invention offers novel equilibration methods for very high resolution, three-dimensional imaging of imaging of lung compliance and distribution of functional residual capacity (FRC) in the lung using hyperpolarized helium-3 (3He) gas (H3He), and collecting local magnetic resonance image data therefrom. Using the present methods permits many functions that have been performed on a regional level for the whole lung using non-polarized helium, to be calculated for the first time from the local MRI measurements of local H3He, such as measuring volume or compliance.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Method and apparatus for determining functional residual capacity of the lungs
The functional residual capacity (FRC) value of a subject's lungs determined for each breath is related a volume dilution factor kn and the final FRC value is determined at the point where the volume dilution factor kn reaches a predetermined volume dilution factor value. The determination of the final FRC value can be based on interpolation or extrapolation from a measured breath data series. The volume dilution factor may be normalized value. This is achieved by division of all volume dilution factors with the initially determined volume dilution factor. With normalization, the compartments in which gas concentration change is achieved slowly are referenced with the faster, more effective compartments, thus giving a ventilation profile of the lungs.
Owner:GENERAL ELECTRIC CO
Apparatus for calculating residual capacity of secondary battery
ActiveUS8965722B2High precision computingWide voltage rangeBatteries circuit arrangementsMaterial analysis by electric/magnetic meansElectrical batteryEngineering
An apparatus for calculating a residual capacity of a secondary battery is provided. The apparatus calculates the residual capacity of energy in the secondary battery which is charged / discharged. The apparatus includes an arithmetic unit which estimates and calculates a first residual capacity based on a charge / discharge voltage corresponding to a residual capacity of the secondary battery, calculates a second residual capacity based on an integrated value of a charge / discharge current of the secondary battery, weights the charge / discharge voltage of the secondary battery with the first residual capacity or the second residual capacity according to the voltage changing rate, and combines the results of the weighting to obtain the residual capacity of the secondary battery.
Owner:DENSO CORP
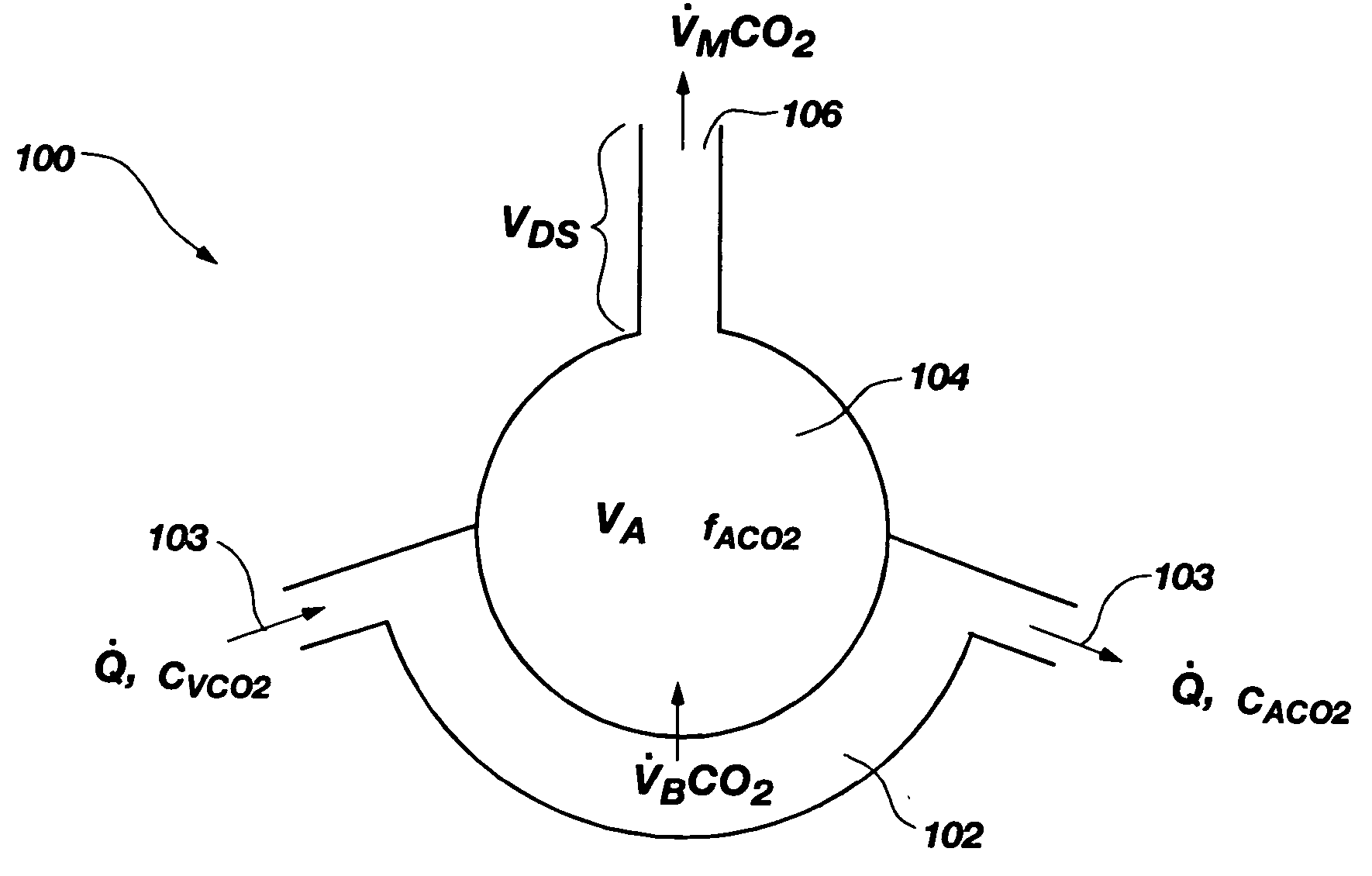
Method and system for estimating the efficiency of the lungs of a patient
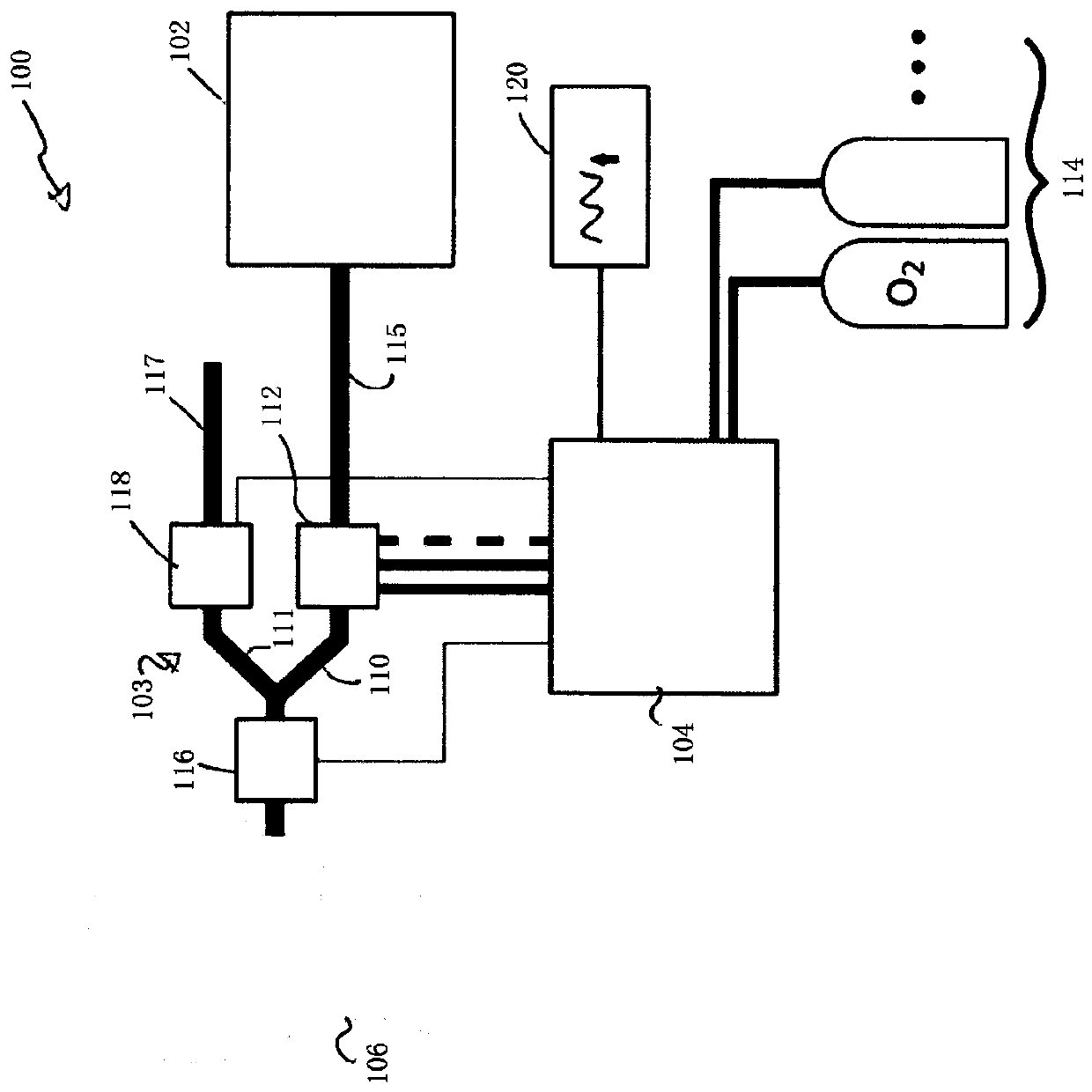
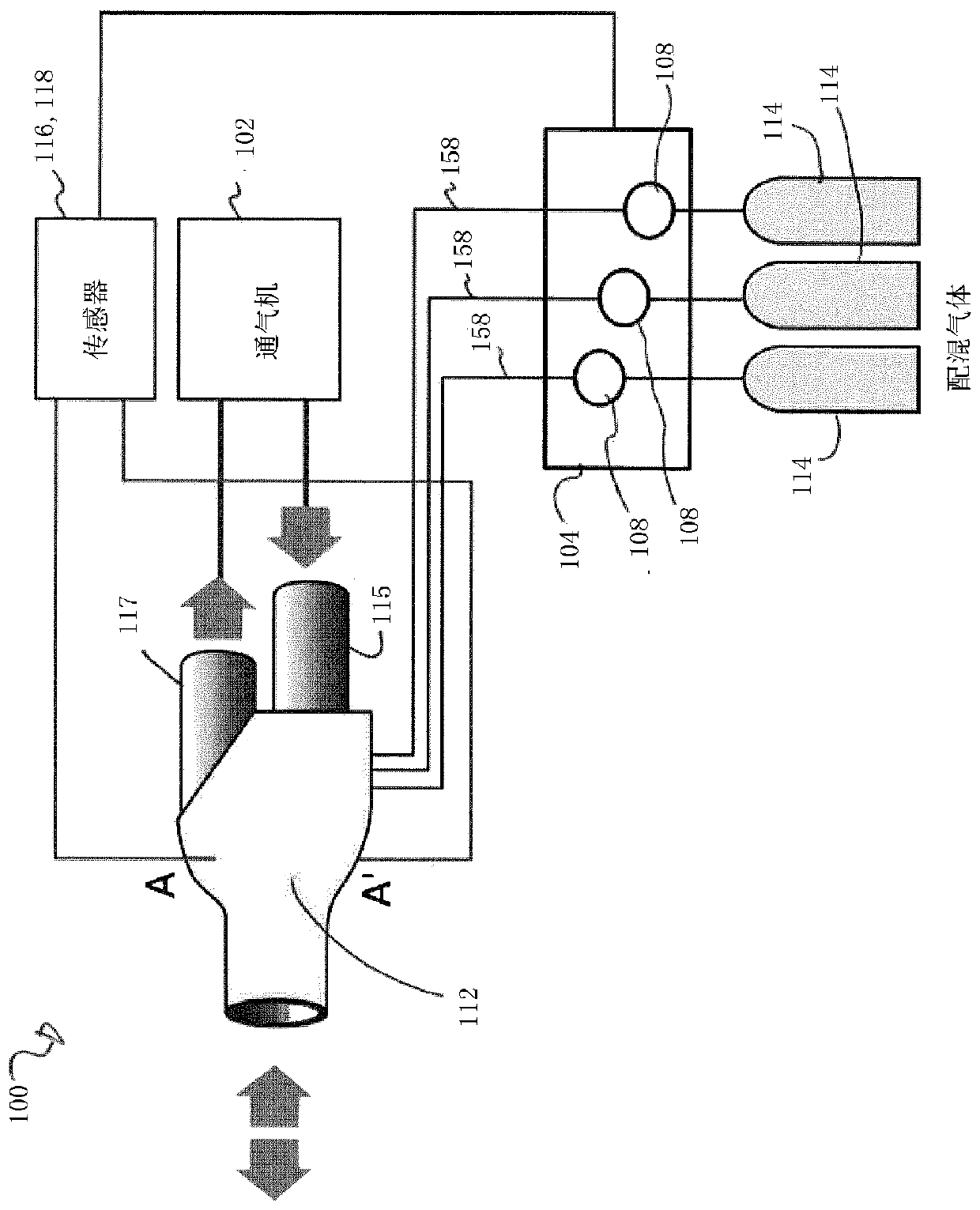
The present disclosure relates to methods and systems for estimating an efficiency of lungs of a patient receiving respiratory care. A blender has a primary input port for receiving a first gas to bedelivered to the patient and one or more secondary input ports for receiving a second gas to be delivered to the patient from one or more gas sources. A patient-side port of the blender delivers the first and second gases to the patient. A gas composition sensor measures a fraction of the first gas and a gas flow sensor measures a flow of the first gas. A controller causes a sequential delivery ofthe first and second gases to the patient and estimates a functional residual capacity of the patient based on measurements from the gas composition sensor and from the gas flow sensor. The controller may also estimate a cardiac output of the patient.
Owner:ROSTRUM MEDICAL INNOVATIONS
Spirometer system and method for determining lung functional residual capacity (FRC) with a non-occluding shutter
InactiveUS20160198980A1Overcome deficienciesDiagnostic signal processingRespiratory organ evaluationEngineeringSpirometer (device)
Owner:TEL HASHOMER MEDICAL RES INFRASTRUCTURE & SERVICES +2
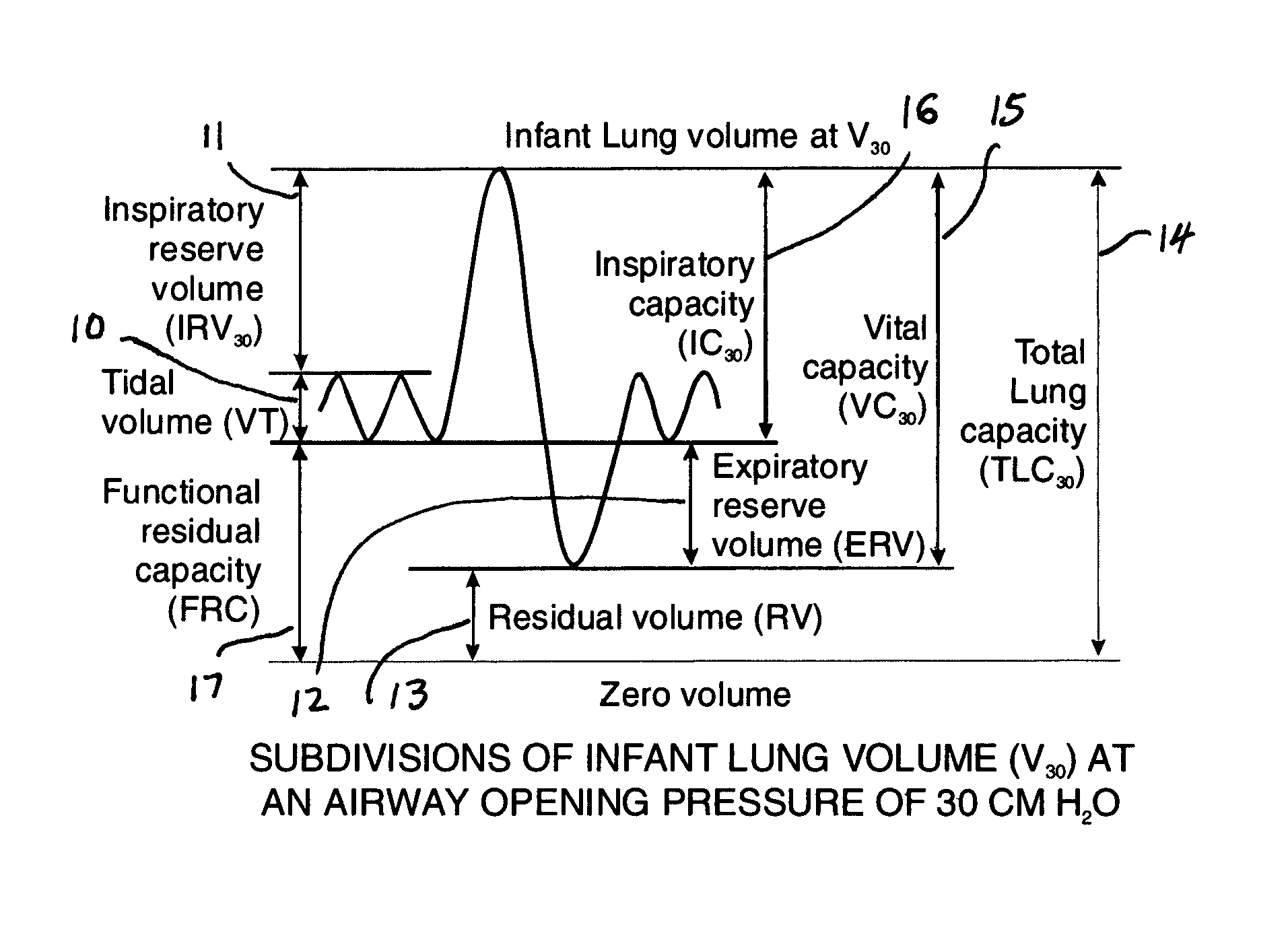
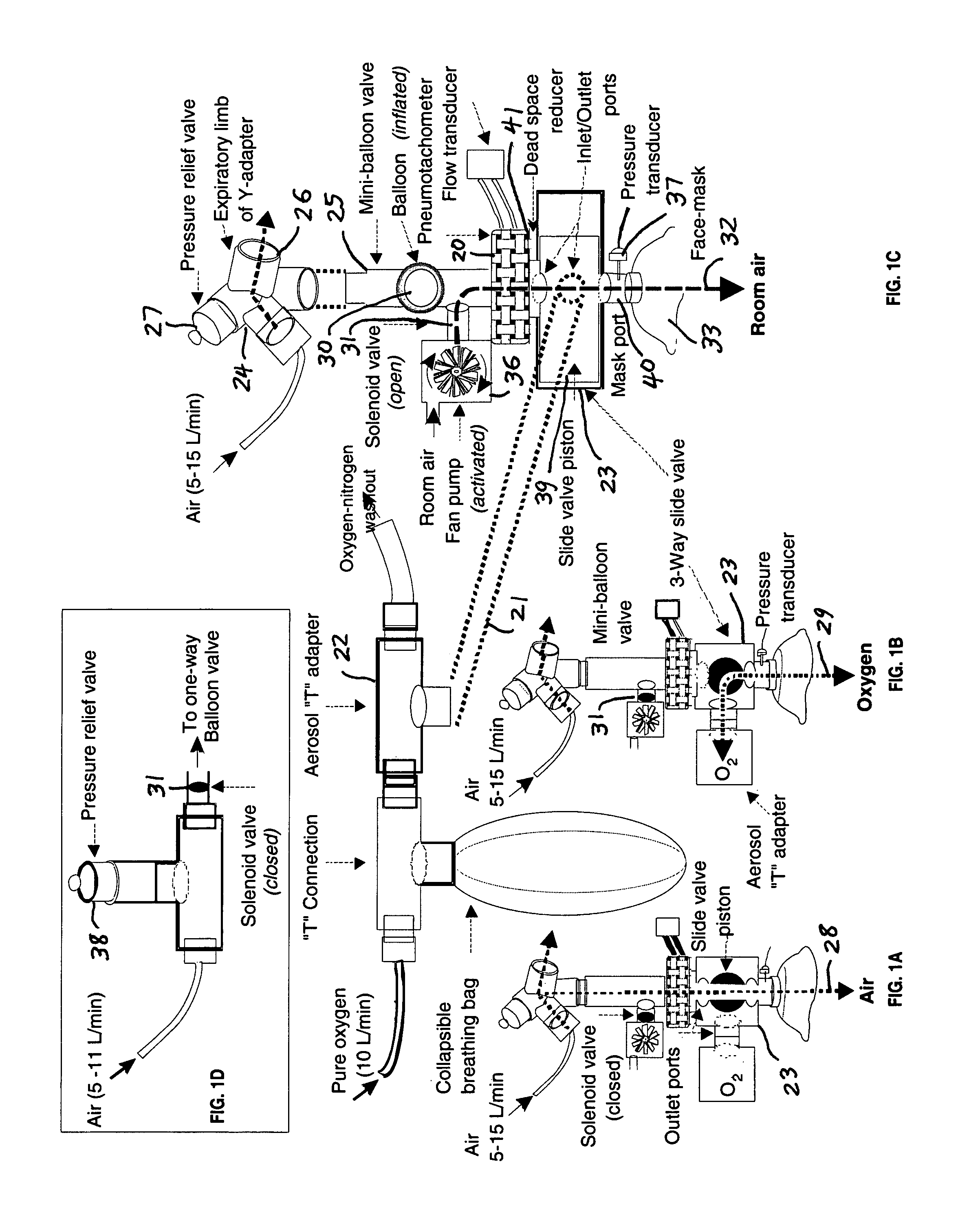
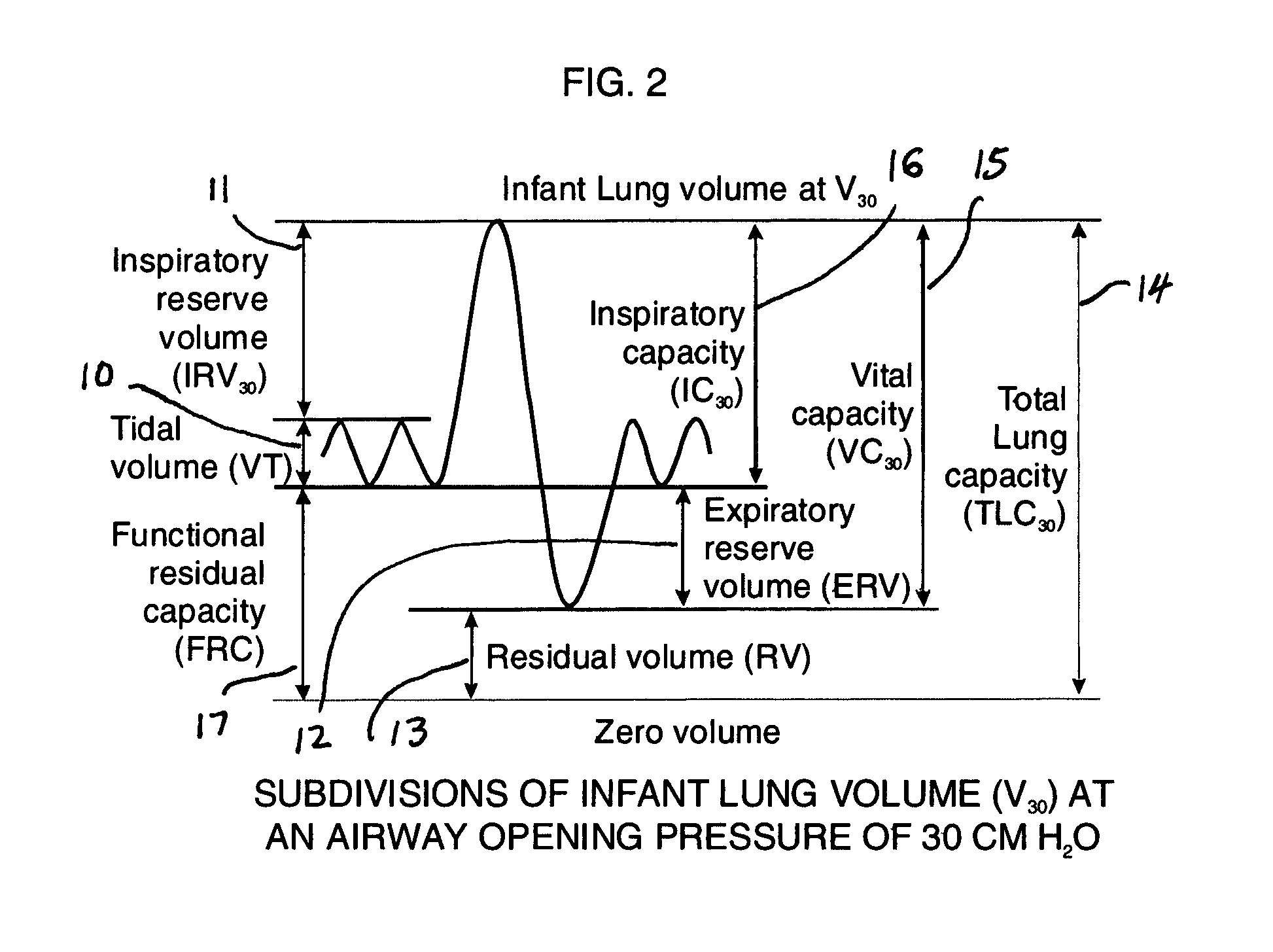
Comprehensive integrated testing protocol for infant lung function
InactiveUS20090131811A1Highly repeatable robust measurementRespiratory organ evaluationSensorsNitrogen gasSlow vital capacity
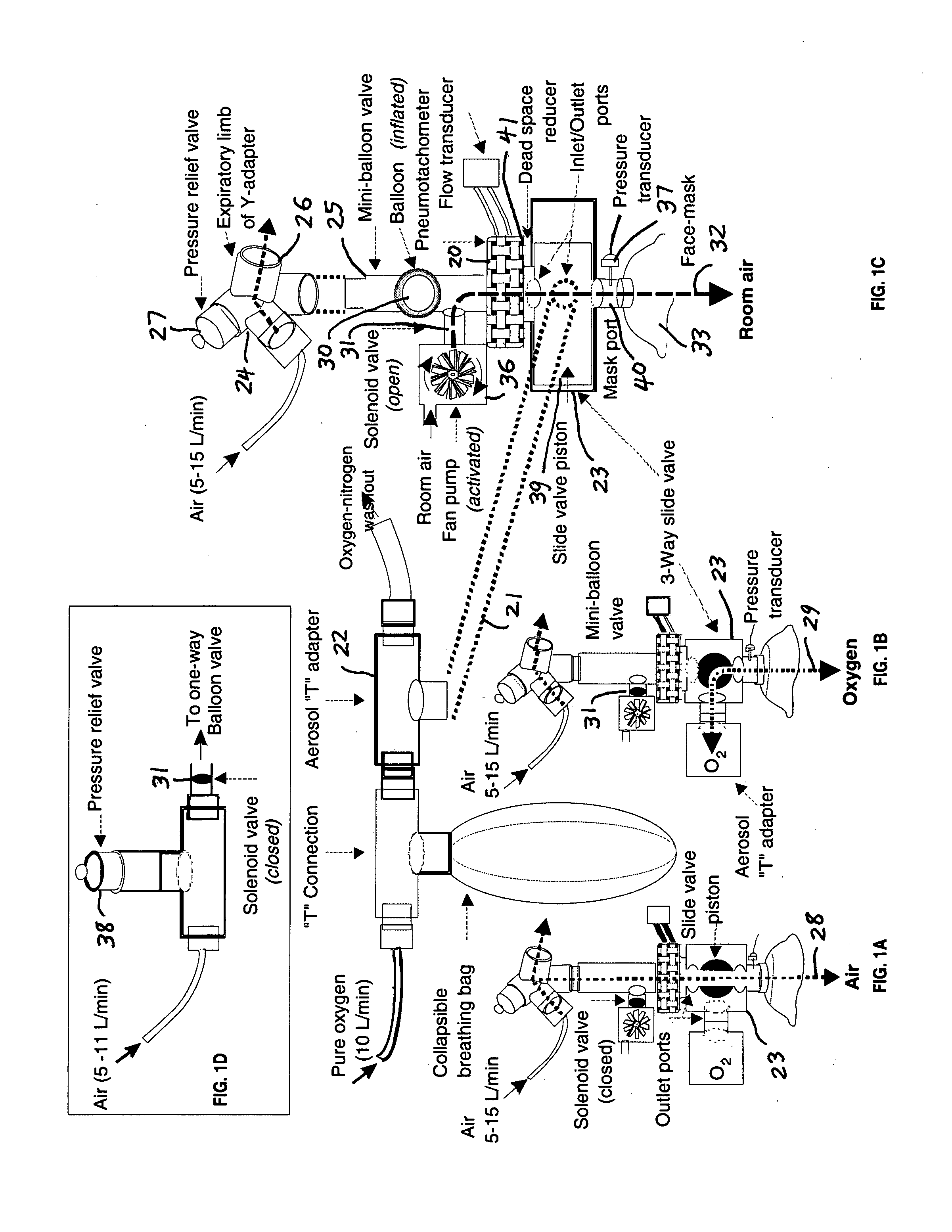
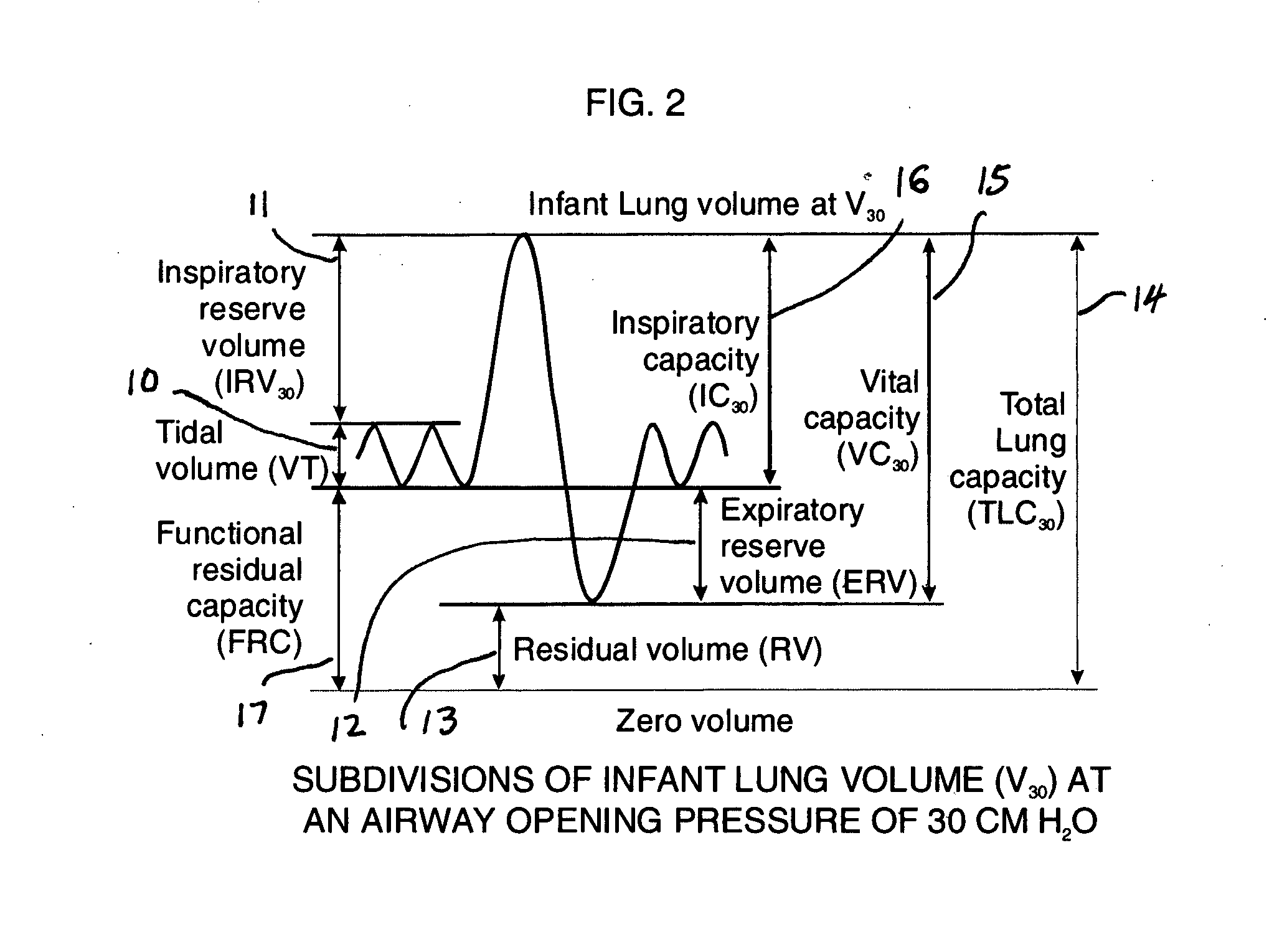
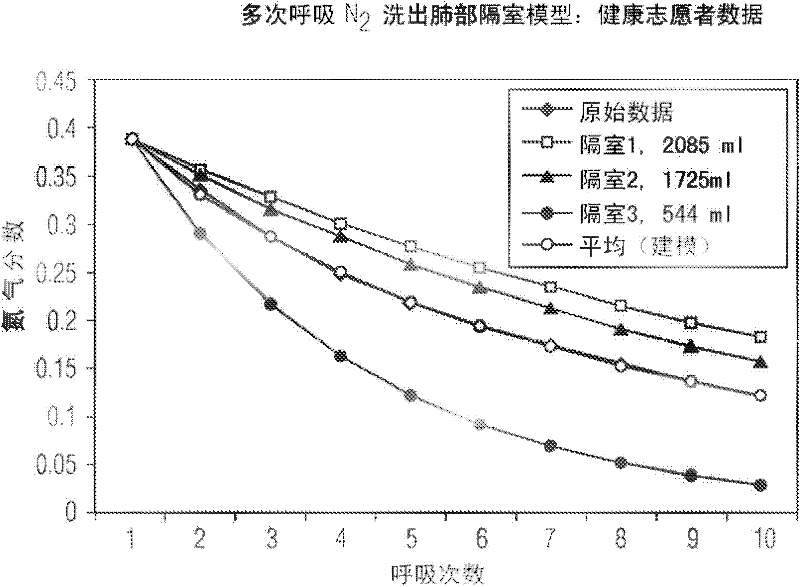
A Comprehensive Integrated Testing Protocol (CITP) incorporates precise measurements of the dynamic and the static lung volumes and capacities at V30 for routine infant lung function testing. The static functional residual capacity (sFRC) in infants is measured after a short hyperventilation induces a post-hyperventilation apnea (PHA) that abolishes the infant's breathing strategies and creates a reliable volume landmark. A measurement of the sFRC is then obtained by inert gas washout; e.g., by measuring the volume of nitrogen expired after end-passive expiratory switching of the inspired gas from room air to 100% oxygen during the PHA. A true measurement of the total lung capacity (TLC) is obtained from the sum of (1) the passively exhaled gas volume from a Pao plateau of 30 cm H2O through a pneumotachometer (PNT) by integrating the flow signal to produce volume, which is the inspiratory capacity (IC), and (2) the sFRC. From intrasubject TLC and residual volume (RV), the difference is a reliable estimate of the slow vital capacity (SVC). Similar measurements may be obtained with a fastened squeeze jacket for comparison. Actual airway opening pressure (aPao) is measured during a 0.20 s airway occlusion after halting the inflating airflow and prior to activating the jacket inflation. An open mouth is maintained during forced expiration in order to generate an oronasal instead of a forced expiration.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS +1
Pulmonary Expansion Therapy (PXT) Devices
ActiveUS20170196762A1Increasing functional residual capacityImprove ventilationRespiratorsPneumatic massageFunctional disturbancePulmonary compliance
A pulmonary expansion therapy (PXT) device may be a handheld device that covers specific lung fields and may generate negative pressure fields locally. The device also may provide vibratory / percussion therapy for airway clearance. The PXT may generate a localized negative pressure field non-invasively to the exterior of the chest wall, thereby increasing the functional residual capacity in underlying lung fields. As a result, increased ventilation and perfusion to the targeted internal lung field may be achieved by creating a decrease in the external barometric pressure relative to the more positive intrinsic airway pressures. The PXT device also may improve lung compliance by enabling a medical professional to grab and elevate the chest wall to compensate for the dysfunction of the respiratory musculature responsible for lifting the chest wall. In some embodiments, once a targeted functional residual capacity (FRC) has been established, vibration or percussion may be applied.
Owner:DELTA DYNAMICS LLC
Determining the functional residual capacity of a subject
A system and method that determine the functional residual capacity of a subject in an automated manner. The determination of the functional residual capacity of the subject is made by analyzing the washout and / or wash-in of one or more molecular species present in gas breathed by the subject. The determination of the functional residual capacity can be made without a determination of oxygen consumption.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Method and apparatus for monitoring a functional capacity of an individual
Owner:WELCH ALLYN INC
Determining the functional residual capacity of a subject
Provided are a system and a method that determine the functional residual capacity of a subject in an automated manner. The determination of the functional residual capacity of the subject is made by analyzing the washout and / or wash-in of one or more molecular species present in gas breathed by the subject. The determination of the functional residual capacity can be made without a determination of oxygen consumption.
Owner:KONINK PHILIPS ELECTRONICS NV
Method of measuring absolute lung volume based on O2/CO2 gas analysis
InactiveUS20060096591A1Low priceRespiratorsOperating means/releasing devices for valvesGas analysisNitrogen
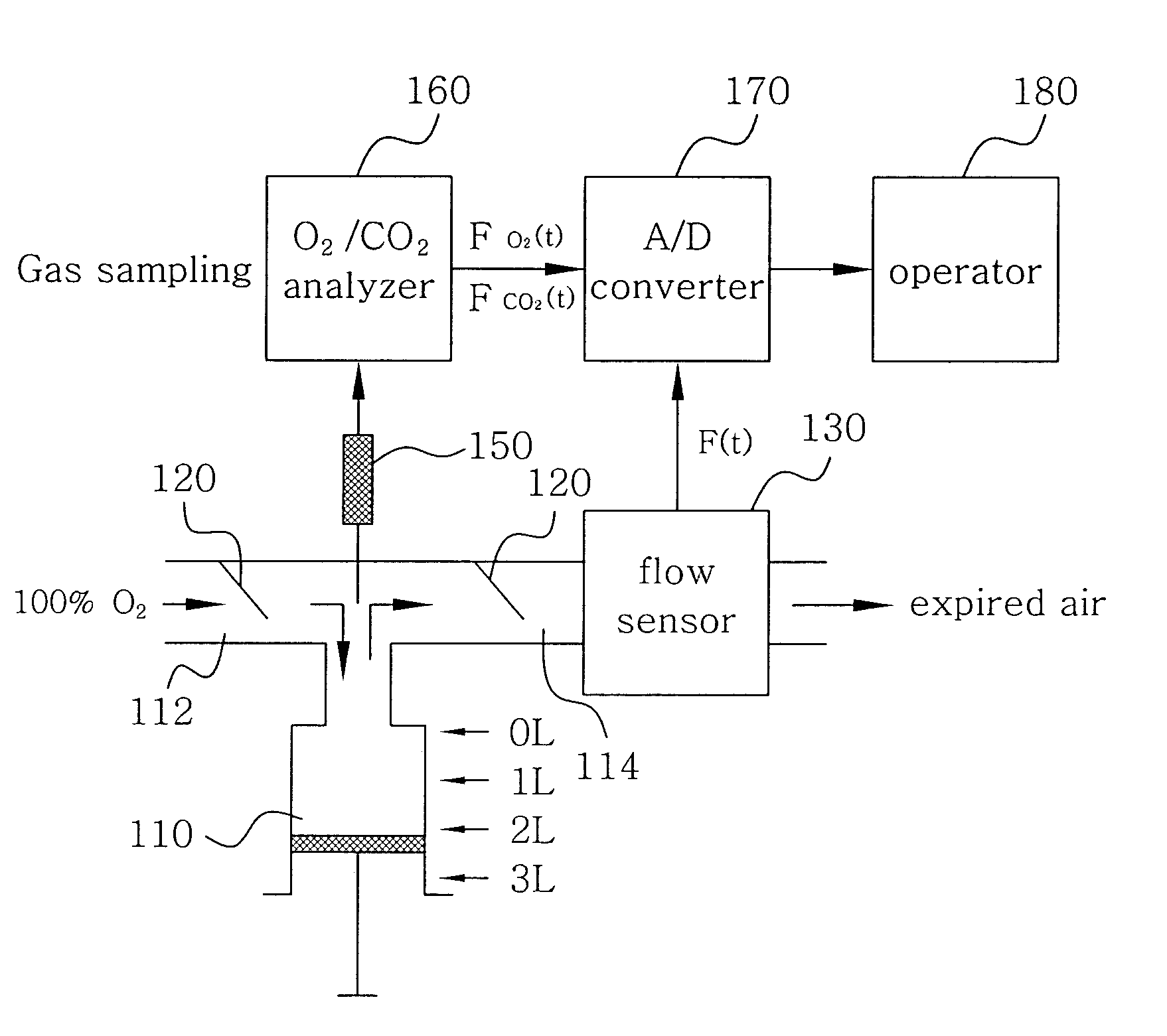
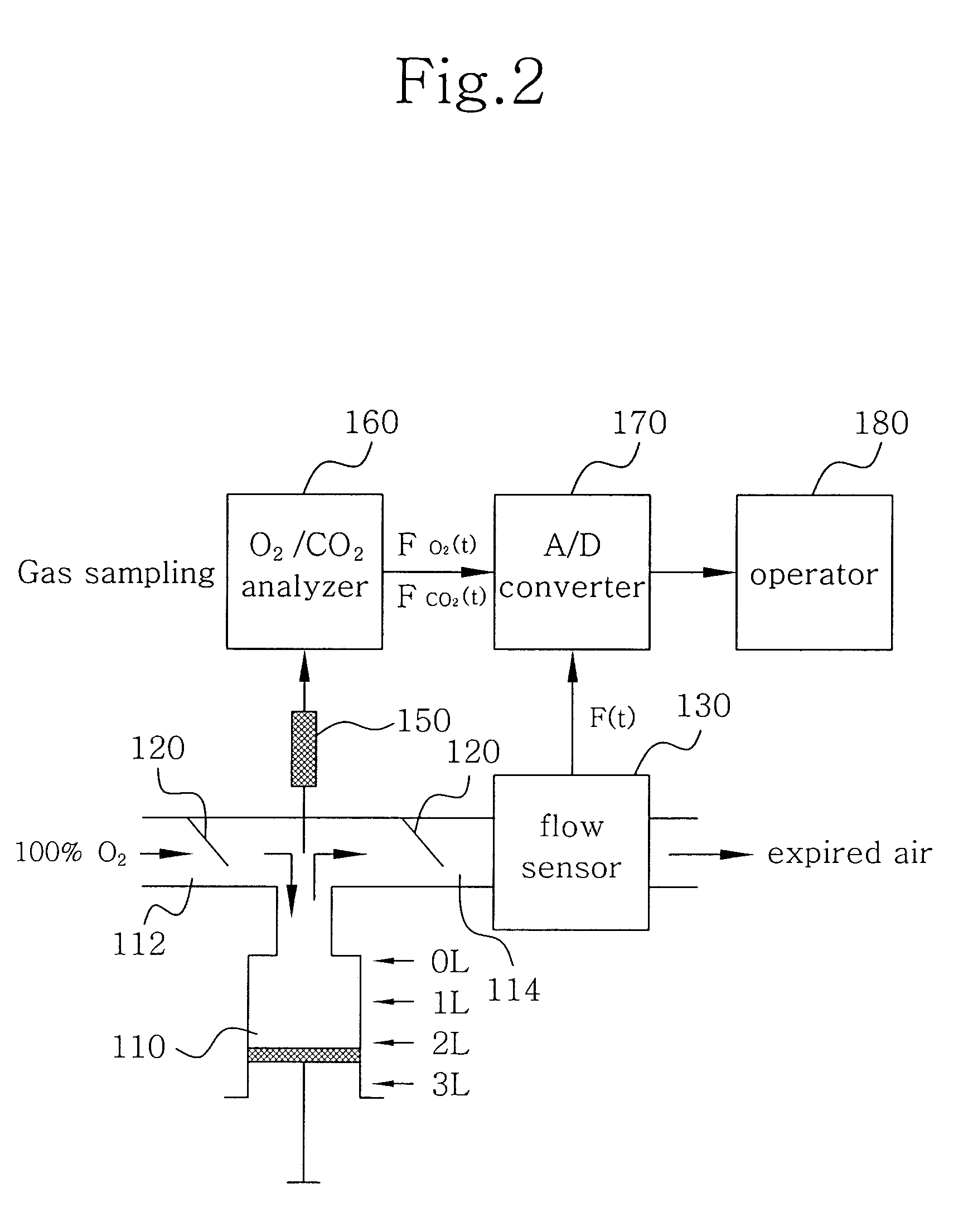
Disclosed herein is a method of measuring an absolute lung volume. In the method, concentrations of oxygen and carbon dioxide gases are measured and analyzed in respiratory gas, consisting of nitrogen (N2), oxygen (O2), and carbon dioxide (CO2), to indirectly measure a concentration of nitrogen, thereby achieving measurement of absolute lung volume, such as a functional residual capacity. The method comprises connecting a one-way valve to a subject so as to separate an inspiration path from an expiration path, measuring a flow rate of respiratory gas using a flow sensor, which is provided in the expiration path, continuously measuring a concentration (FO2) of oxygen and a concentration (FCO2) of carbon dioxide using O2 and CO2 sensors provided in the expiration path, correcting dynamic characteristics of the concentration (FO2) of oxygen and the concentration (FCO2) of carbon dioxide so that the dynamic characteristics agree with each other in terms of time, and analyzing oxygen and carbon dioxide gases using the following Equation. FRC=10.79∫(1-(FO2-FCO2))Fⅆt
Owner:CHUNGBUK NAT UNIV IND ACADEMIC COOPERATION FOUND
Comprehensive integrated testing protocol for infant lung function
InactiveUS8246550B2Highly repeatable robust measurementRespiratory organ evaluationSensorsForced expirationSlow vital capacity
A Comprehensive Integrated Testing Protocol (CITP) incorporates precise measurements of the dynamic and the static lung volumes and capacities at V30 for routine infant lung function testing. The static functional residual capacity (sFRC) in infants is measured after a short hyperventilation induces a post-hyperventilation apnea (PHA) that abolishes the infant's breathing strategies and creates a reliable volume landmark. A measurement of the sFRC is then obtained by inert gas washout; e.g., by measuring the volume of nitrogen expired after end-passive expiratory switching of the inspired gas from room air to 100% oxygen during the PHA. A true measurement of the total lung capacity (TLC) is obtained from the sum of (1) the passively exhaled gas volume from a Pao plateau of 30 cm H2O through a pneumotachometer (PNT) by integrating the flow signal to produce volume, which is the inspiratory capacity (IC), and (2) the sFRC. From intrasubject TLC and residual volume (RV), the difference is a reliable estimate of the slow vital capacity (SVC). Similar measurements may be obtained with a fastened squeeze jacket for comparison. Actual airway opening pressure (aPao) is measured during a 0.20 s airway occlusion after halting the inflating airflow and prior to activating the jacket inflation. An open mouth is maintained during forced expiration in order to generate an oronasal instead of a forced expiration.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS +1
Assessment of concentration of inhalational compounds in the brain
This invention directs to a method of assessing the concentration of an inhalational compound in the brain of a subject. The method includes administering a gas containing the compound into a subject to fill the pulmonary functional residual capacity, which is defined as the remaining lung volume at the end of an unforced respiration. The method also includes measuring an inspired compound concentration (Ci′) and an expired compound concentration (Ce′) after having filled the functional residual capacity with the gas. Therefore, a mixed venous compound concentration (Cb′) can be assessed based on the formula Cb′=[Ci′(M−1)+Ce′] / M, in which M is an alveolar membrane factor for the compound; and a brain compound concentration (Cb) can be assessed based on the formula Cb=(Ce′+2Cb′) / 3 or Cb=(Ce′+3Cb′) / 4.
Owner:LIN CHUNG YUAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com