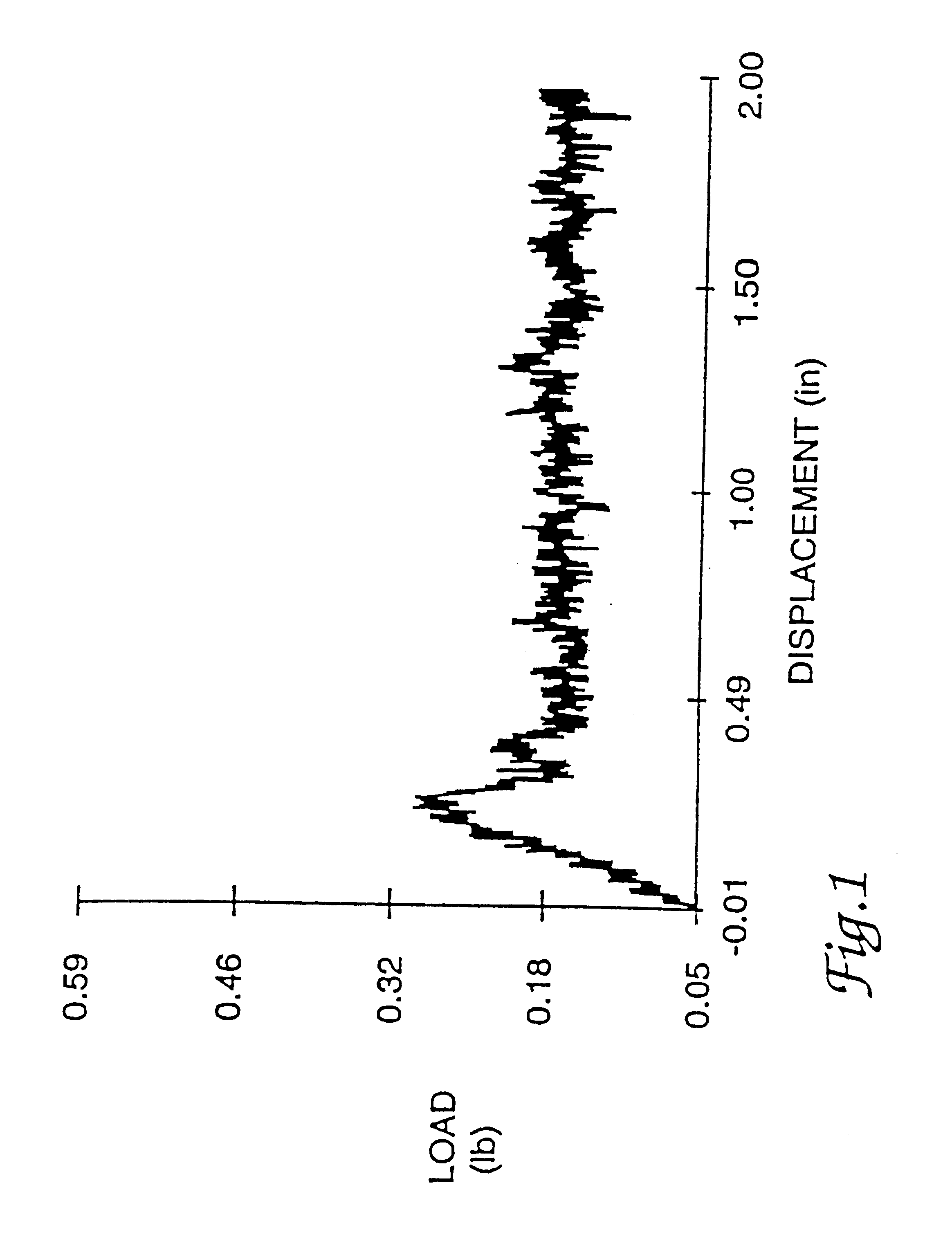
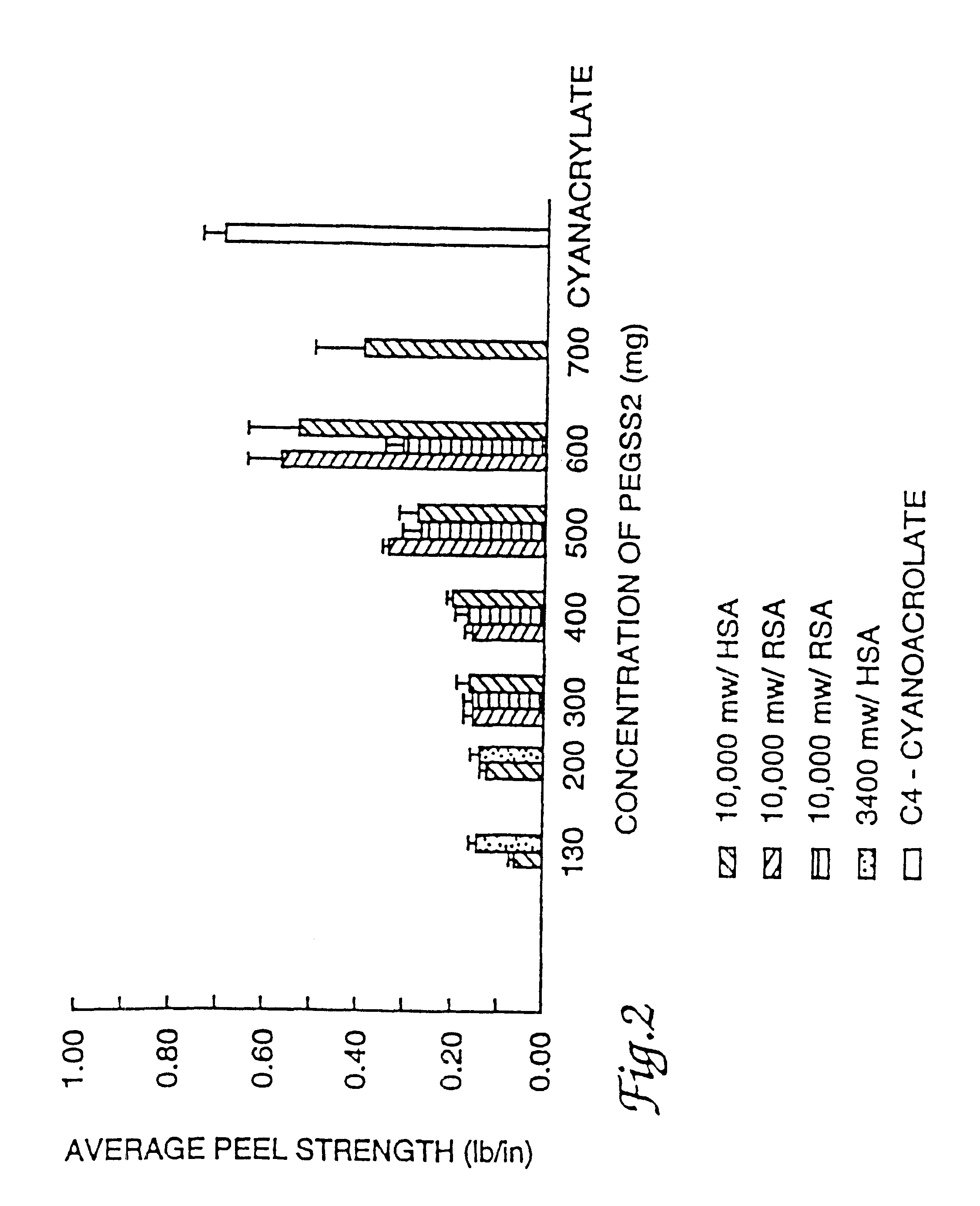
Adhesive sealant composition
a technology of adhesive sealant and composition, which is applied in the field of adhesive sealant composition, can solve the problems of limited adhesive composition, unsuitable tissue adhesive or sealant, and inability to meet the needs of paper after-treatment,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Polyethylene Glycol Disuccinimidyl Succinate PEG-SS2
Polyethylene glycol, PEG, (50 g, Aldrich Chemical Company, Milwaukee, Wis., sold as 3,400 average molecular weight, GPC analysis M.sub.n was 2,980, M.sub.w, was 3,480) was dissolved in 1,2-dichloroethane (250 ml) containing succinic arthydride (14.7 g) and anhydrous pyridine (12 ml). The mixture was refluxed under nitrogen for three days. After filtration and evaporation of the solvent, the residue was dissolved in 100 ml water and treated with the cation exchange resin Dowex.TM. 50 (H.sup.+) (50 g) for 30 minutes. The mixture was then filtered and the Dowex.TM. 50 was washed with water (50 ml 1.times.). The combined filtrate was washed with anhydrous diethyl ether (50 ml 2.times.). The PEG-disuccinate was then extracted from the water phase with two 100 ml chloroform washes. Evaporation of chloroform yielded about 49 g of PEG-disuccinate.
The PEG-disuccinate was dissolved in 200 ml N,N-dimethylformamide (DMF) at 37.deg...
example 2
Synthesis of N-hydroxysuccinimide Ester of Dicarboxymethyl Polyethylene Glycol
Dicarboxymethyl poly(ethylene glycol) (mol. wt. 3400) purchased from Shearwater Polymers, Inc., Huntsville, Ala. (5 g) and N-hydroxysuccinimide purchased from Sigma Chemical Co., St. Louis, Mo. (1 g) were dissolved in 30 ml of anhydrous DMF with mechanical stirring under nitrogen. The solution was cooled to 0.degree. C. and a solution of dicyclohexylcarbodiimide (1.79 g) in 5 ml DMF was added dropwise. The stirring was continued in the cold for 3 hours then at room temperature overnight (16 hrs). Dicyclohexylurea which precipitated was removed by filtration. Toluene (100 ml) was added to the filtrate and cooled to 0.degree. C. The product was then precipitated by addition of petroleum ether. The precipitate was collected on a sintered glass filter. Dissolution in toluene and reprecipitation with petroleum ether was repeated three times. The product was dried under vacuum in a desiccator.
example 3
Synthesis of Polyethylene Glycol-di-oligoglycolide Disuccinimidyl Succinate
A 500 ml three neck round bottom flask was flame dried under nitrogen. 50 g of PEG (mol. wt. 3400), 300 ml of xylene, and 1 drop of 0.33M stannous ottoate solution in xylene were charged into the flask with a continuous nitrogen purge. The flask was heated to boil the solution and 50 ml of xylene were removed by distillation. The solution was then cooled to room temperature. 17 g of glycolide (Boehfinger Ingleheim KG, Ingleheim, Germany) was added to the flask and the reaction mixture was refluxed under nitrogen for 16 hours. The copolymer reaction mixture was filtered hot to remove polyglycolide homopolymer. The copolymer then precipitated from the filtrate upon cooling and collected by filtration. The copolymer was placed in a flask with 500 ml of dichloromethane and 7 g of succinyl chloride. The solution was refluxed under nitrogen overnight (16 hours). 8.5 g of N-hydroxysuccinimide was added to the flask ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Linear density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com