Over expression of foldases and chaperones improves protein production
a foldase and protein technology, applied in the field of overexpression of foldases and chaperones, can solve the problems of high overexpression and poorly secreted, and achieve the effect of increasing protein secretion and increasing secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Vector for Over-Expression of bip1 in T. reesei
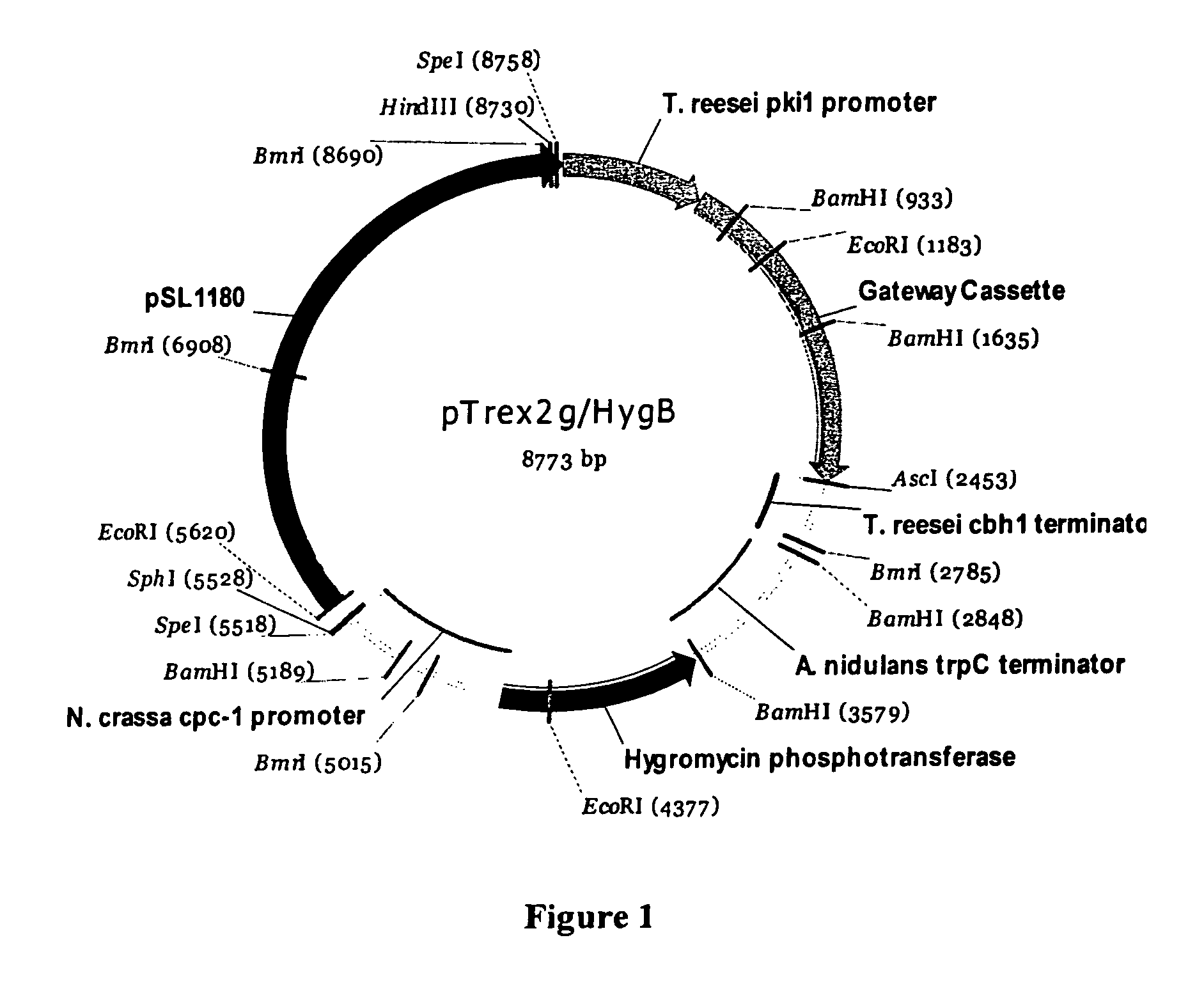
[0055]A Gateway-compatible expression vector, pTrex2g / hygB, was designed to enable over-expression of the T. reesei chaperone gene bip1. After insertion into pTrex2g / hygB the open reading frame of the bip1 gene was flanked by the promoter sequences of the T. reesei pki1 gene and the terminator sequences of the T. reesei cbh1 gene. The vector also contained the E. coli hygromycin phosphotransferase (hph) gene flanked by the promoter sequences of the Neurospora crassa cpc-1 gene and the terminator sequences of the Aspergillus nidulans trpC gene.
[0056]The following segments of DNA were assembled in the construction of Trex2g / HygB (see FIG. 1):
[0057]A 728 bp fragment of T. reesei genomic DNA representing the promoter region from the pki1 (pyruvate kinase) gene. At the 5′ end of this DNA were 6 bp of synthetic DNA representing a SpeI restriction site and at the 3′ end were 6 bp of synthetic DNA adding a SacII restriction site.
[0058]The 1714...
example 2
The Trichoderma reesei Chymosin Production Strain CHY1-2
[0062]A synthetic version of the bovine prochymosin B open reading frame (see FIG. 2, SEQ ID NO: 42) was constructed with codon usage optimized for expression in Trichoderma. A vector, pTrex4-ChyGA was designed for the expression of an open reading frame encoding a fusion protein that consists of the following components from the amino-terminus: the T. reesei CBHI secretion signal sequence, the T. reesei CBHI catalytic core and linker region, and the bovine prochymosin B protein. This open reading frame is flanked by the promoter and terminator sequences of the T. reesei cbh1 gene. The vector also contains the Aspergillus nidulans amdS gene, encoding acetamidase, as a selectable marker for transformation of T. reesei.
[0063]The following segments of DNA were assembled in the construction of pTrex4-ChyGA (see FIG. 3):
[0064]The T. reesei cbh1 promoter and coding region. This DNA sequence begins at a naturally occurring HindIII si...
example 3
Cloning the T. reesei bip1 Gene and Insertion into pTrex2g / hygB
[0070]In order to insert the T. reesei bip1 gene into pTrex2g / HygB the DNA sequence was amplified by PCR using attB PCR primers. The forward primer (F-attB1) had the following sequence at the 5′ end, 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCT-3′ (SEQ ID NO:43), followed by a sequence specific to the 5′ end of the bip1 open reading frame. The reverse primer (R-attB2) had the following sequence at the 5′ end, 5′-GGGGACCACTTTGTACAAGAAAGCTGGGT-3′ (SEQ ID NO:44), followed by a sequence specific to the 3′ end of the bip1 open reading frame. The full sequence of the two primers was:
[0071]
(SEQ ID NO: 31)5′-GGGGACAAGTTTGTACAAAAAAAGGCTATGGCTCGTTCACGGAGCTCCC-3′(SEQ ID NO: 32)5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTTACAATTCGTCGTGGAAGTCGCC-3′
[0072]The bip1 gene was amplified using Phusion polymerase from Finnzymes (Cat. No. F-530) according to the manufacturer's directions. The PCR mixture contained 1 μl T. reesei genomic DNA, 10 μl 5× buffer HF, 1 μ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com