Copper CHA zeolite catalysts
a zeolite catalyst and copper cha technology, applied in the field of zeolites, can solve the problems that the activity of many metal-promoted zeolites begins to decline, and achieve the effects of improving the nh3 scr of nox, excellent hydrothermal stability, and reducing nitrogen oxides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
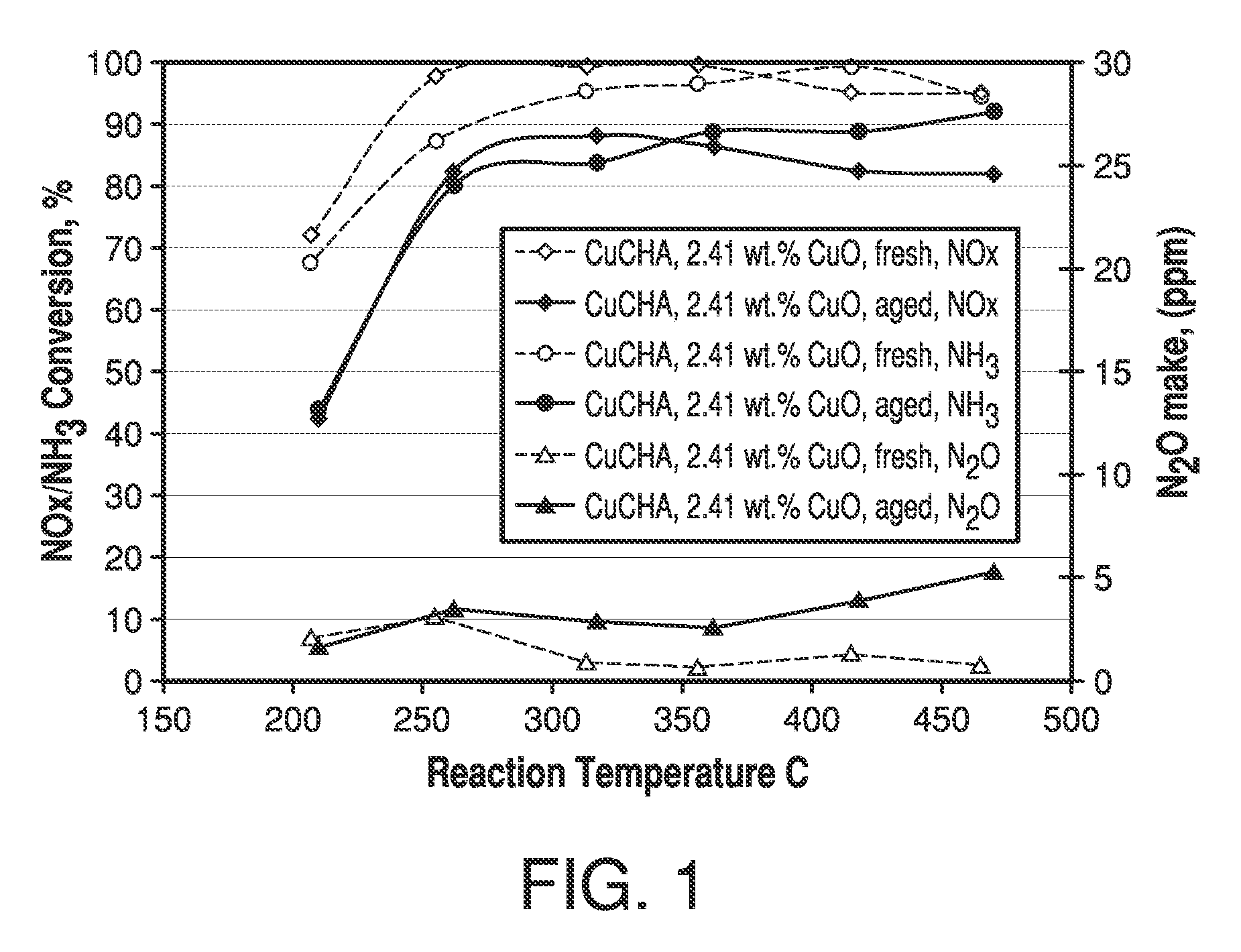
[0062]A CuCHA powder catalyst was prepared by mixing 100 g of NH4+-form CHA, having a silica / alumina mole ratio of 30, with 400 mL of a copper(II) sulfate solution of 1.0 M. The pH was adjusted to 3.5 with nitric acid. An ion-exchange reaction between the NH4+-form CHA and the copper ions was carried out by agitating the slurry at 80° C. for 1 hour. The resulting mixture was then filtered, washed with 800 mL of deionized water in three portions until the filtrate was clear and colorless, which indicated that substantially no soluble or free copper remained in the sample, and the washed sample was dried at 90° C. The above process including the ion-exchange, filtering, washing and drying was repeated once.
[0063]The resulting CuCHA product was then calcined at 640° C. in air for 6 hours. The obtained CuCHA catalyst comprised CuO at 2.41% by weight, as determined by ICP analysis. A CuCHA slurry was prepared by mixing 90 g of CuCHA, as described above, with 215 mL of deionized water. Th...
example 1a
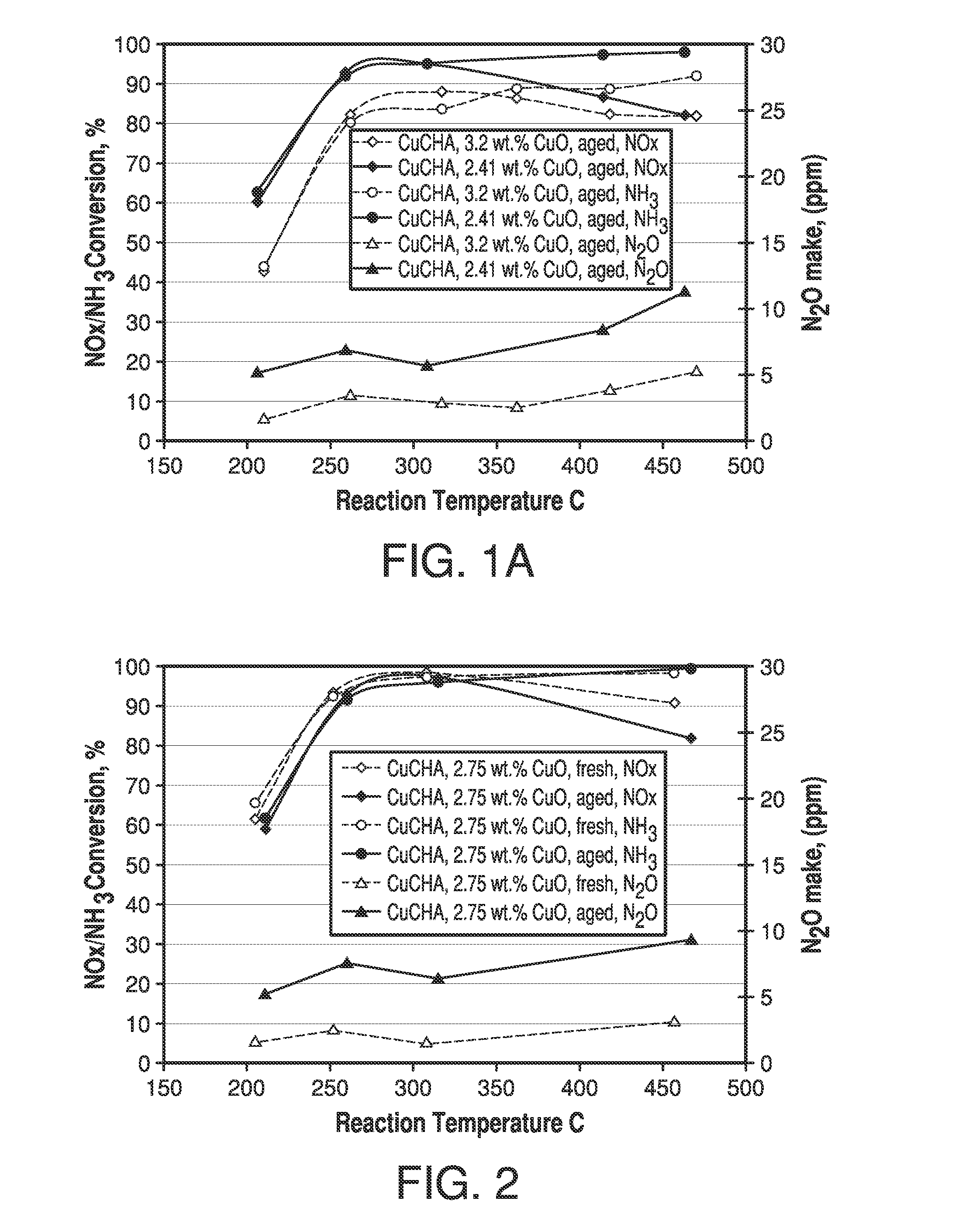
[0068]To the coating slurry of Example 1 was added copper sulphate pentahydrate to bring up the total CuO level to 3.2%. The slurry was coated onto monolith and aged and tested for SCR NOx as outlined above for Example 1, except that the monolith was calcined at 640° C. The catalytic performance was compared with Example 1 in FIG. 1A. The addition of copper sulphate into the coating slurry significantly improved the hydrothermal stability and low temperature activity.
example 2
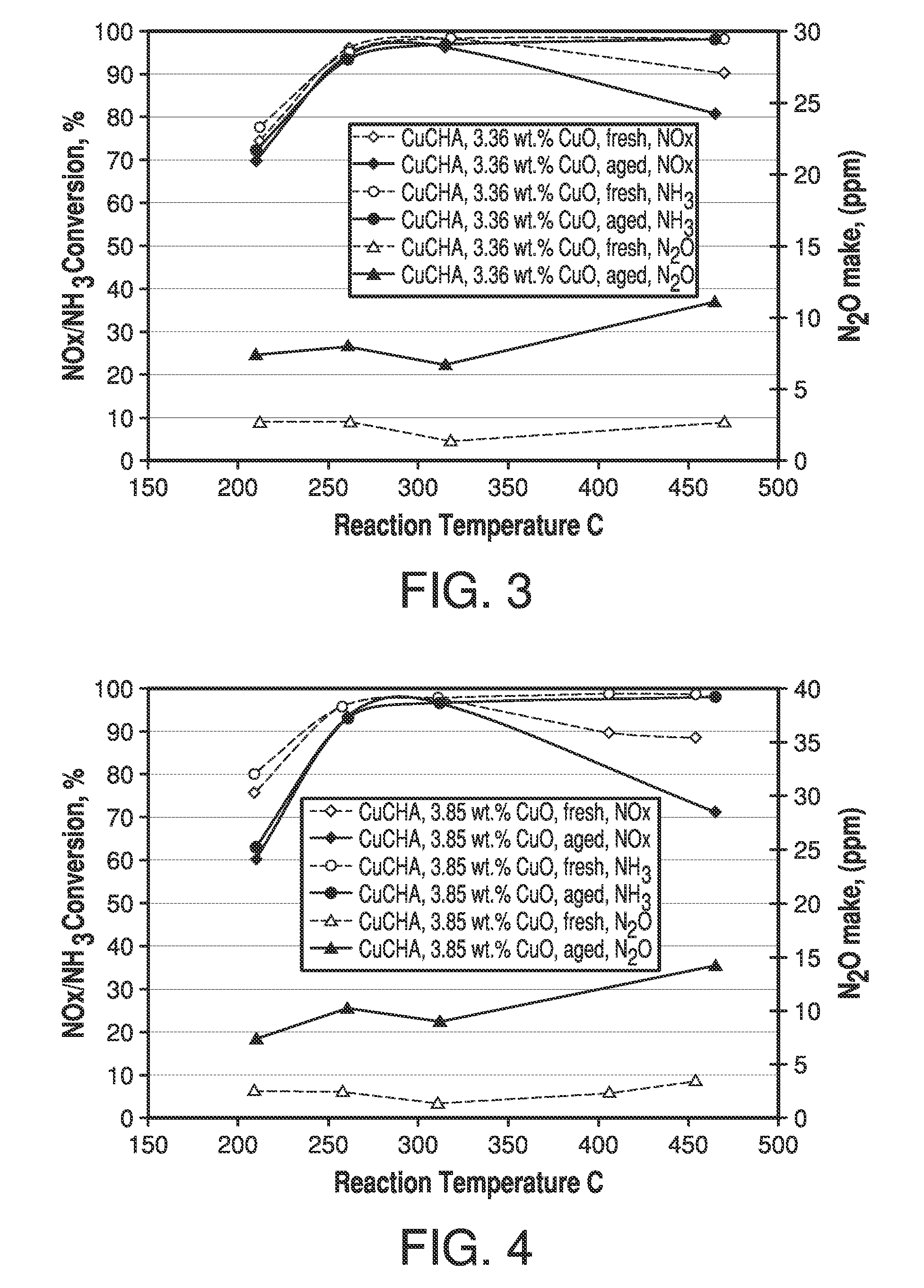
[0069]A CuCHA powder catalyst was prepared by mixing 17 Kg of NH4+-form CHA, having a silica / alumina mole ratio of 30, with 68 L of a copper(II) sulfate solution of 1.0 M. The pH was adjusted to 3.5 with nitric acid. An ion-exchange reaction between the NH4+-form CHA and the copper ions was carried out by agitating the slurry at 80° C. for 1 hour. The resulting mixture was then filtered and air-dried. The above process including the ion-exchange and filtering was repeated once. Then the wet filter cake was reslurried into 40 L deionized water followed by filtering and drying at 90° C. The resulting CuCHA product was then calcined at 640° C. in air for 6 hours. The obtained CuCHA catalyst comprised CuO at 2.75% by weight.
[0070]The slurry preparation, coating and SCR NOx evaluation were the same as outlined above for Example 1. This example contained free copper, and exhibited improved hydrothermal stability compared with Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| mole ratio | aaaaa | aaaaa |
| mole ratio | aaaaa | aaaaa |
| mole ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com