Preparation method and use of Cu-SSZ-13 molecular sieve based catalyst
A cu-ssz-13, molecular sieve technology, applied in molecular sieve catalysts, separation methods, chemical instruments and methods, etc., can solve the problems of inability to effectively control the content of active components, consumption of large purified water, high cost, and achieve excellent catalytic activity. , The effect of strong controllability and high crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 Benzyl quaternary ammonium ion is used as template to prepare Cu-SSZ-13 catalyst
[0027] Sequentially add copper sulfate to deionized water and stir at room temperature for 30 minutes, then add tetraethylenepentamine equal in mass to copper sulfate and a certain amount of organic template, and continue stirring for 3 hours to form a copper amine complex solution. Sodium metaaluminate, sodium hydroxide, and deionized water were mixed and stirred for 1 hour, then mixed with the copper amine complex solution, and stirred for another 4 hours. Finally, silica sol was added to the mixed solution, mixed and stirred for 4 hours to obtain an initial gel. The system Na 2 O, Al 2 o 3 , SiO 2 、H 2 The molar weights of O, copper sulfate-tetraethylenepentamine and organic template are shown in Table 1.
[0028] Put the fully stirred gel in a hydrothermal reaction kettle, react at 180°C for 6 days, cool to room temperature after the reaction is completed, centrifug...
Embodiment 2
[0033] Example 2 N, N, N-trimethyl-1-adamantyl amine cation is used as template to prepare Cu-SSZ-13 catalyst
[0034] The addition order and stirring time of gel preparation, hydrothermal reaction conditions and aftertreatment process are all according to embodiment 1, and this system Na 2 O, Al 2 o 3 , SiO 2 、H 2 The molar weights of O, copper sulfate-tetraethylenepentamine and templating agent are as shown in Table 2.
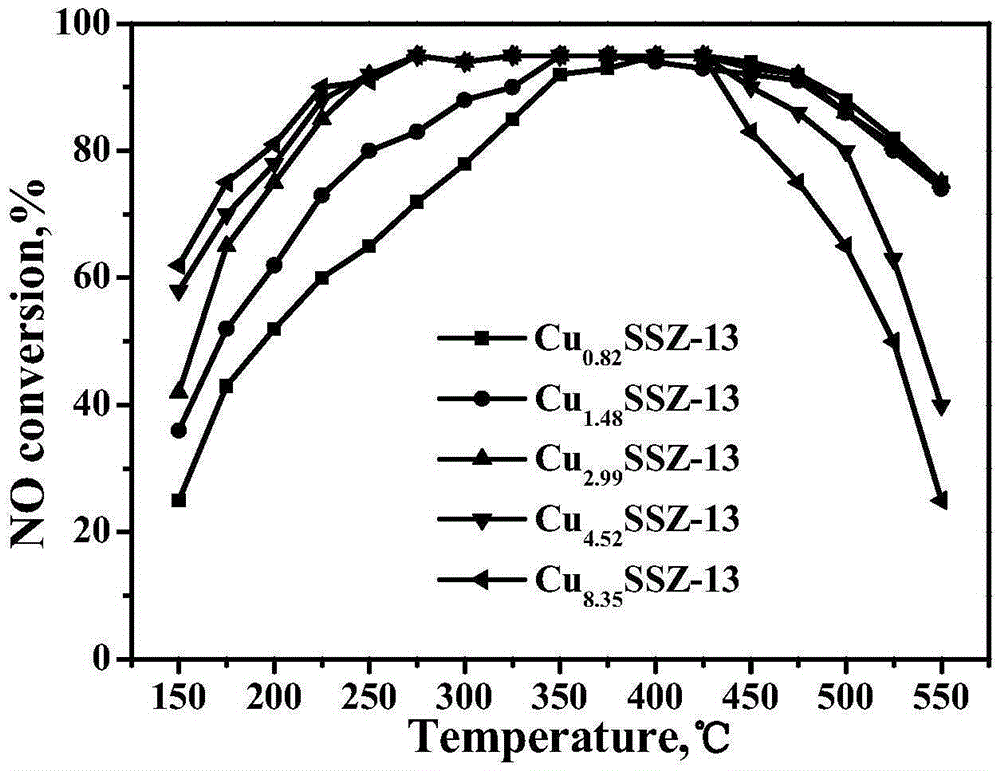
[0035] The Cu-SSZ-13 molecular sieve catalysts obtained by the five groups of experiments in Example 2 were tested by ICP and XRF respectively, and the loadings of the active components were found to be 2.64wt.%, 3.91wt.%, 5.98wt.%, 7.05wt.%, 10.13wt.%. It shows that the catalyst with lower Cu content can still be obtained without any post-treatment by using the template agent, and the Cu content and the silicon-aluminum ratio of the catalyst can be adjusted within a certain range.
[0036] Table 2
[0037]
[0038]
Embodiment 3
[0039] Embodiment 3 choline chloride is used as template to prepare Cu-SSZ-13 catalyst
[0040] The addition order and stirring time of gel preparation, hydrothermal reaction conditions and aftertreatment process are all according to embodiment 1, and this system Na 2 O, Al 2 o 3 , SiO 2 、H 2 The molar weights of O, copper sulfate-tetraethylenepentamine and organic template are shown in Table 3.
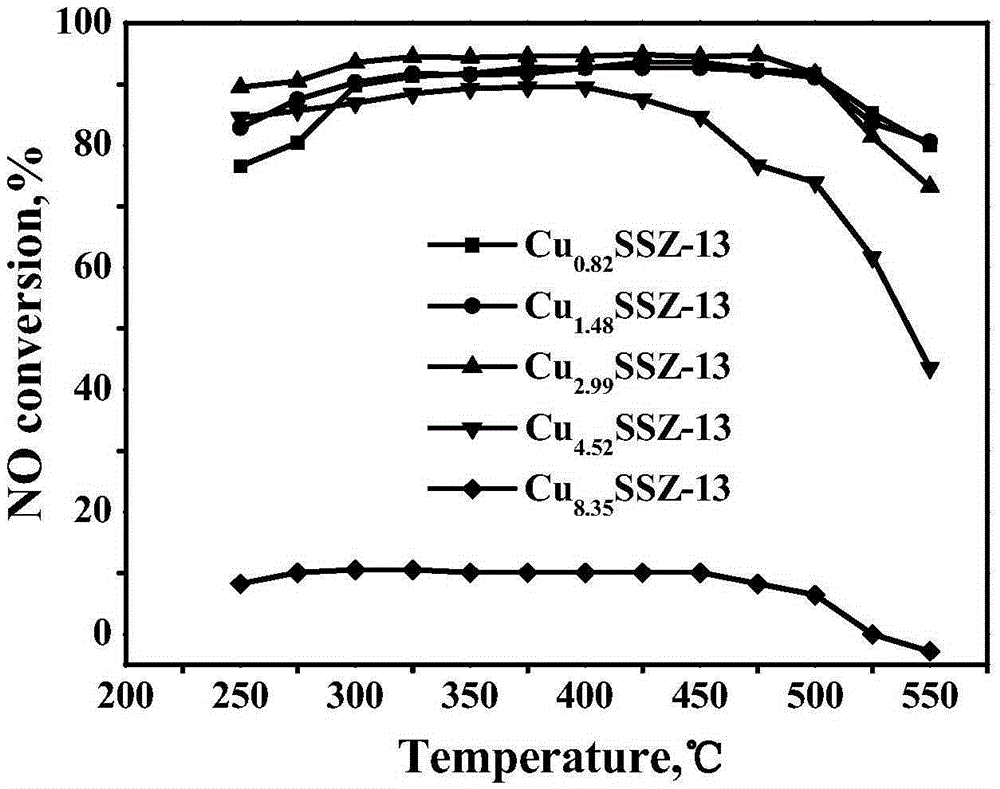
[0041] The Cu-SSZ-13 molecular sieve catalysts obtained in the five groups of experiments in Example 3 were tested by ICP and XRF respectively, and the loadings of the active components were found to be 2.85wt.%, 2.91wt.%, 2.98wt.%, 3.05 wt.%, 3.13wt.%, the ratio of silicon to aluminum is 15.8, 16.2, 16.8, 17.2, 17.9. It shows that using the template agent, the catalyst with lower Cu content can be obtained without post-treatment, and the Cu content and the silicon-aluminum ratio of the catalyst can be adjusted within a certain range.
[0042] table 3
[0043]
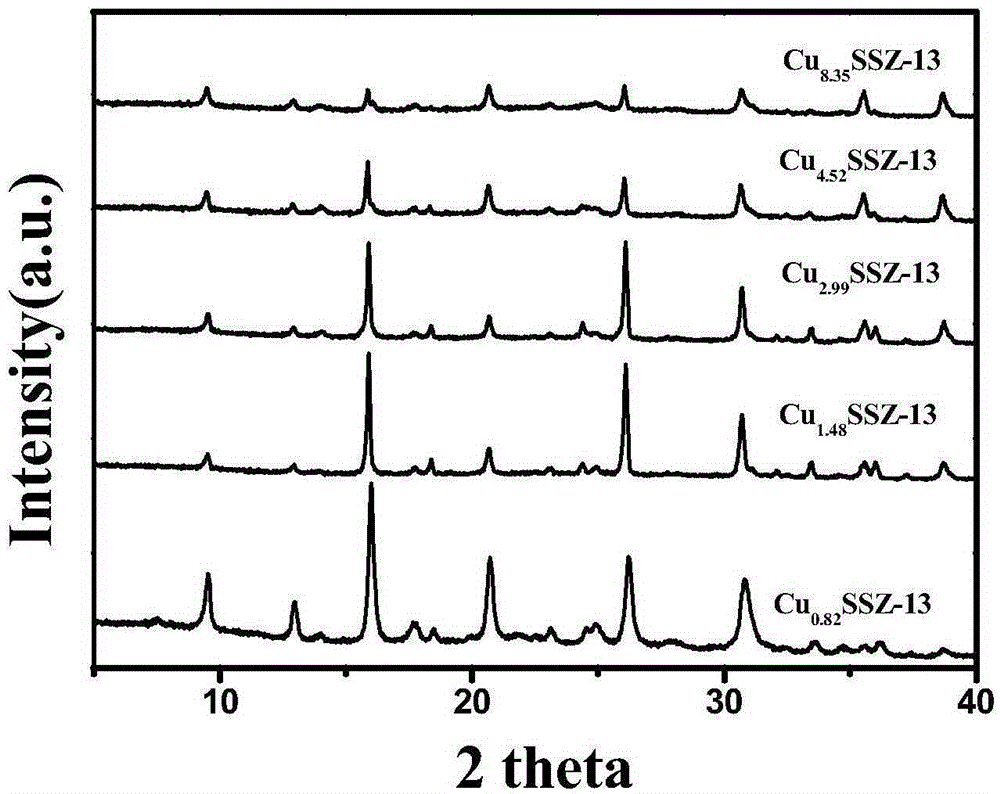
[0044] Fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com