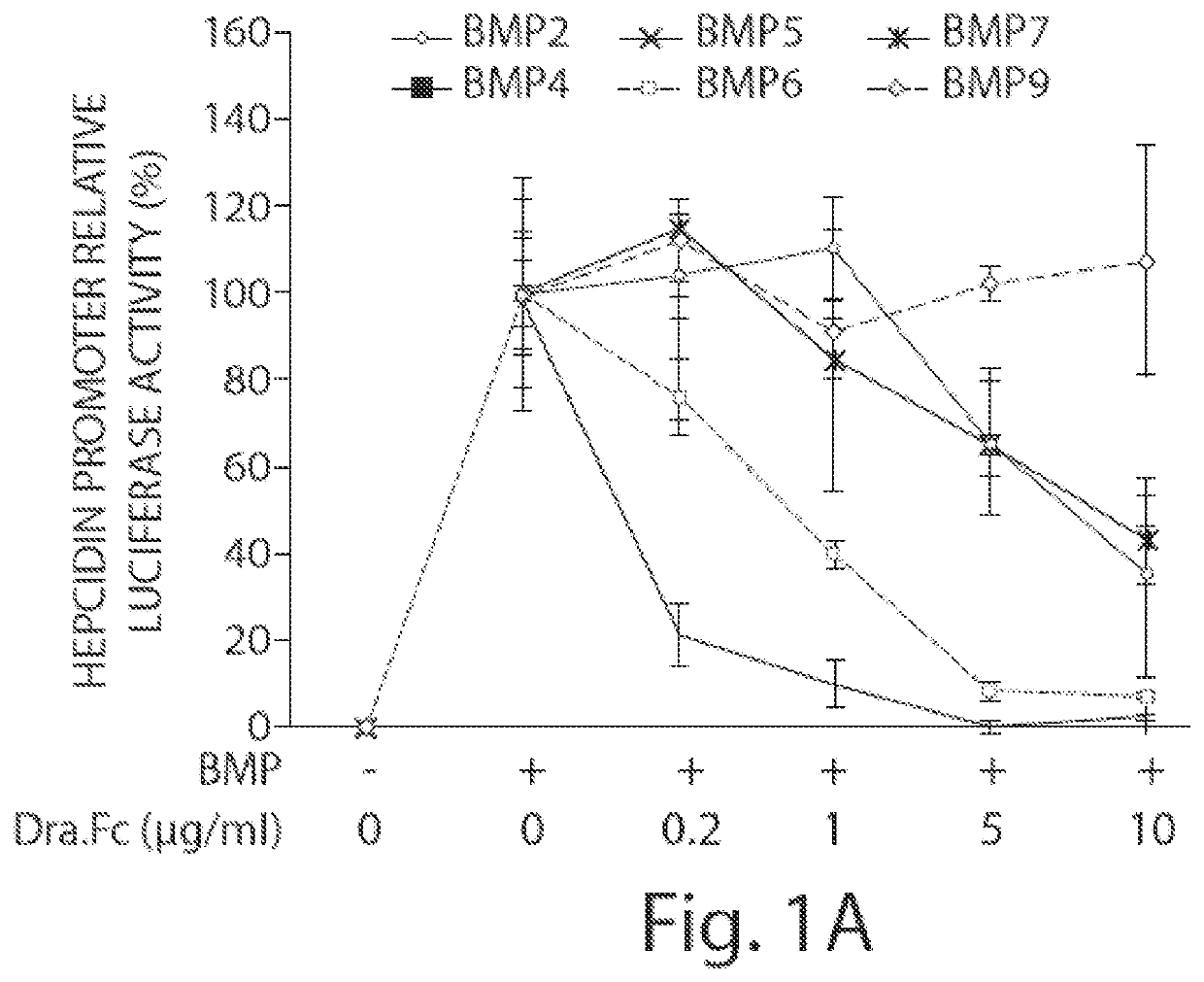
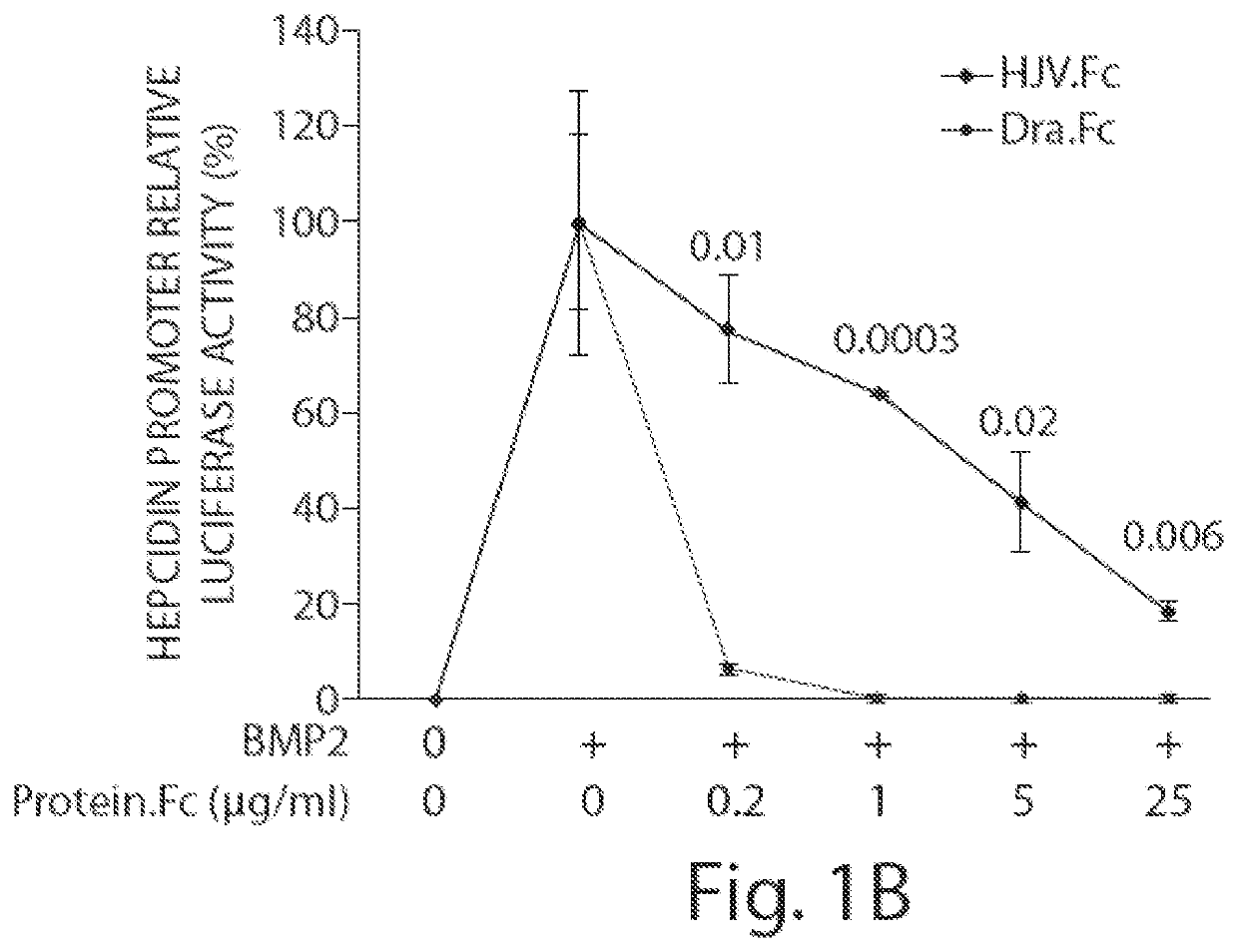
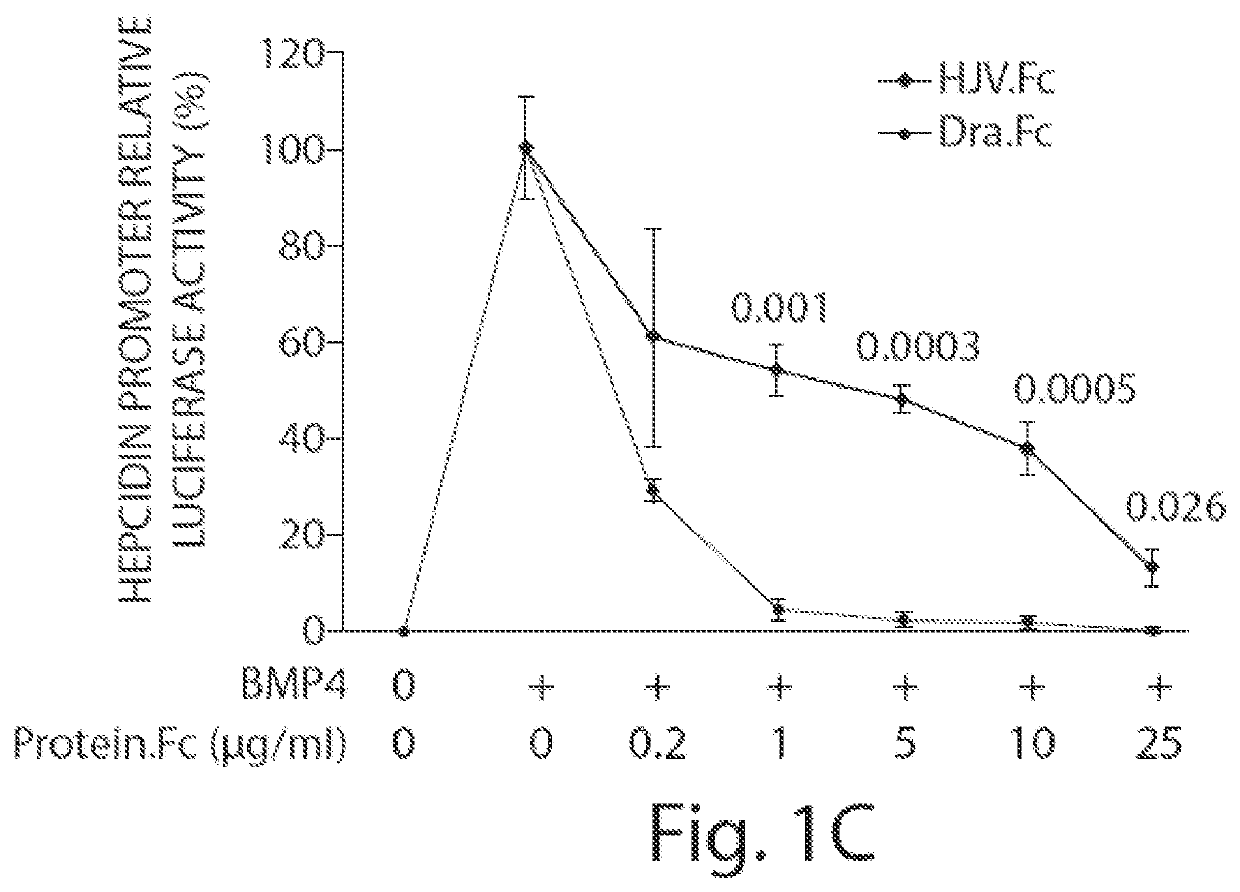
Methods and compositions for regulating iron homeostasis by modulation of bmp-6
a technology of iron homeostasis and bmp-6, which is applied in the field of bmp6 and bmp6 protein specific reagents, can solve the problems of iron deposits in these organs and tissues, and achieve the effects of reducing hepcidin expression, increasing serum iron level, and increasing serum transferrin saturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of cDNA
[0151]cDNA encoding codon optimized DRAGON.Fc was generated by GenScript Corporation (Piscataway, N.J. 08854), based on the human DRAGON protein sequence upstream of the predicted GPI anchor (UniProtKB / Swiss-Prot accession Q6NW40, amino acids 1-409) and the human IgG1 Fc sequence (from the Signal pIg plus vector (R&D Systems) and GenBank AF150959).
example 2
n and Purification of DRAGON.Fc and HJV.Fc
[0152]cDNA encoding DRAGON.Fc was transfected using 293fectin (Invitrogen) into Freestyle 293-F cells (Invitrogen) according to the manufacturer's instructions. Transfected cells were cultured in GIBCO Freestyle 293 Expression medium (Invitrogen) shaking at 110 RPM in a humidified 8% CO2 incubator at 37° C. Seven days after transfection, cells were pelleted by centrifugation and DRAGON.Fc was purified from the media via one-step Protein A affinity chromatography using Hi-Trap rProtein A FF columns (Amersham Biosciences) as described in Babbitt, J. L., et al. 2005. J. Biol. Chem. 280:29820-29827. HJV.Fc was produced as described in Babitt, J. L., et al. 2007. J Clin Invest. 117:1933-9. To determine purity and to quantify protein concentration, DRAGON.Fc and HJV.Fc were subjected to reducing SDS-PAGE followed by Bio-safe Coomassie blue staining (Bio-Rad) as well as Western blot with anti-HJV antibody (Babitt, J. L., et al. 2006. Nat. Genet. 38...
example 3
n of BMP-6
[0153]Purified recombinant human BMP-6 was prepared as previously described. (Simic, P., et al. 2006. J Biol Chem. 281:25509-21). Lyophilized BMP-6 was dissolved in 20 mM sodium acetate, 5% mannitol solution, pH 4.0 for animal injections.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Electrical conductance | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com