Synthetic peptide sp2 and application thereof
a synthetic peptide and peptide technology, applied in the field of biomedicine, can solve the problems of cardiovascular toxicity doing more harm to cancer patients, poor efficacy and survival of single-agent chemotherapy, adverse reactions in patients at effective doses, etc., and achieve significant dose-effect and time-effect relationship, prevent the occurrence of tumor metastasis, and prevent the effect of toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0058]Amino acid composition, solid-phase chemical synthesis, chromatographic purification and mass spectrometry identification
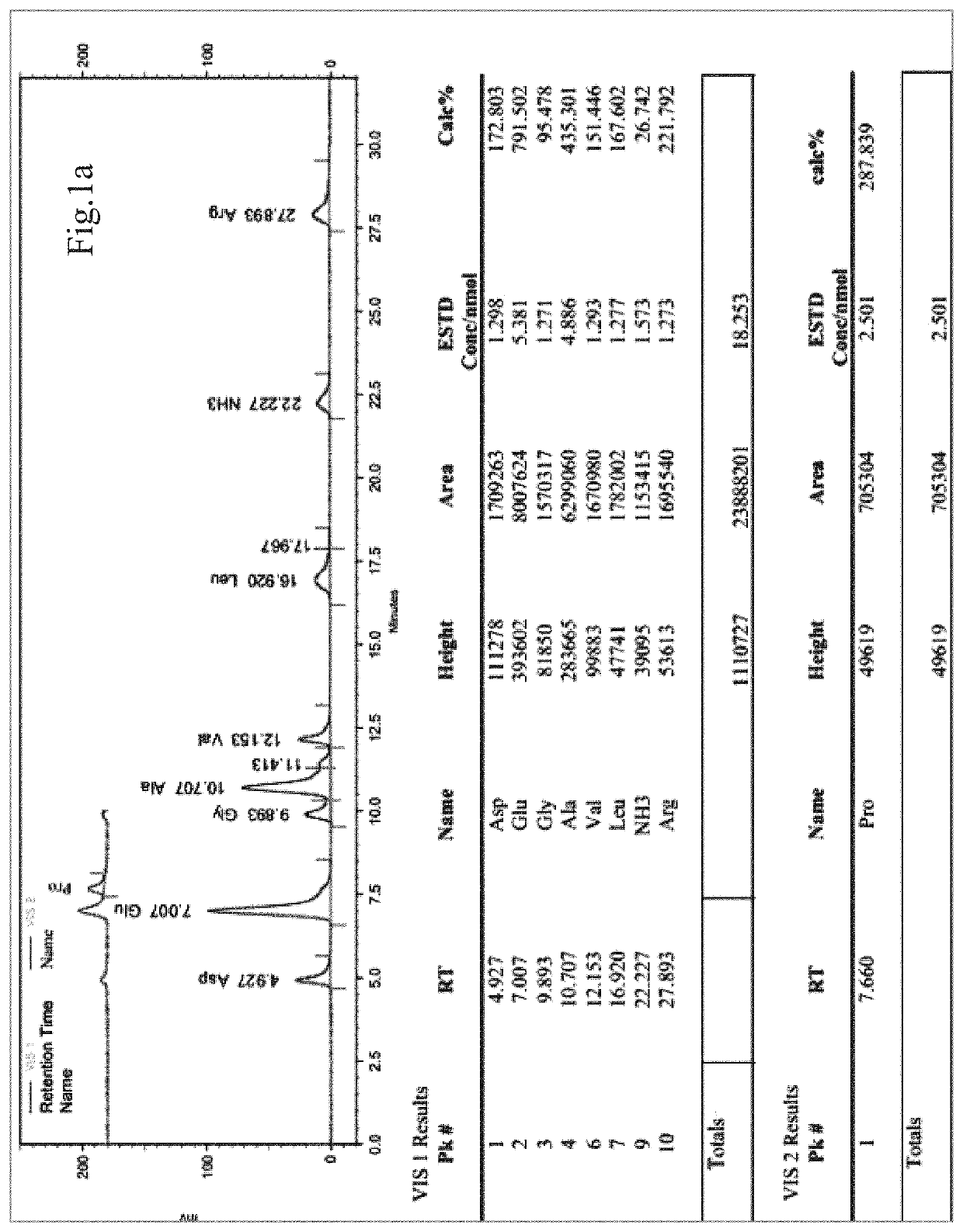
[0059]1) Analysis of the amino acid composition of sp2: 10.2 mg sample was weighed and was dissolved with 7 mL of 6N HCl, and hydrolyzed at 110° C. under nitrogen protection for 22 hours. The reaction solution was transferred to a 10 mL volumetric flask after cooling, and was made to volume. 0.2 mL of the solution was taken and blown dry with nitrogen at 55° C.; 1 mL of distilled water was added and dried, and repeated three times. The dried product was dissolved thoroughly with 1.2 mL of deionized water (pH was adjusted with 0.02 mol / L HCl) and mixed well. It was filtered with 0.45 μM filter membrane, and injected 20 μL for computer testing (Hitachi L-8900 amino acid analyzer).
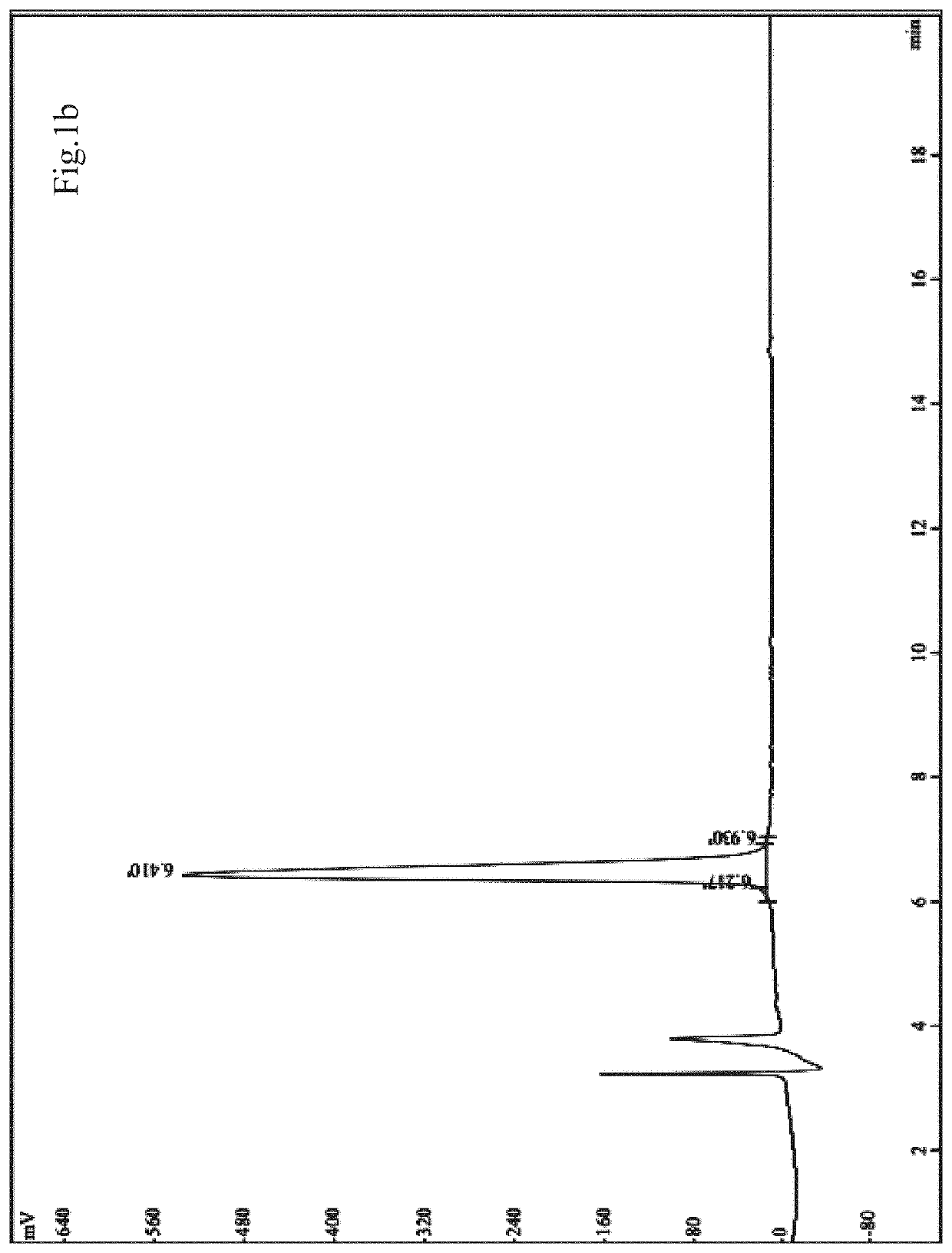
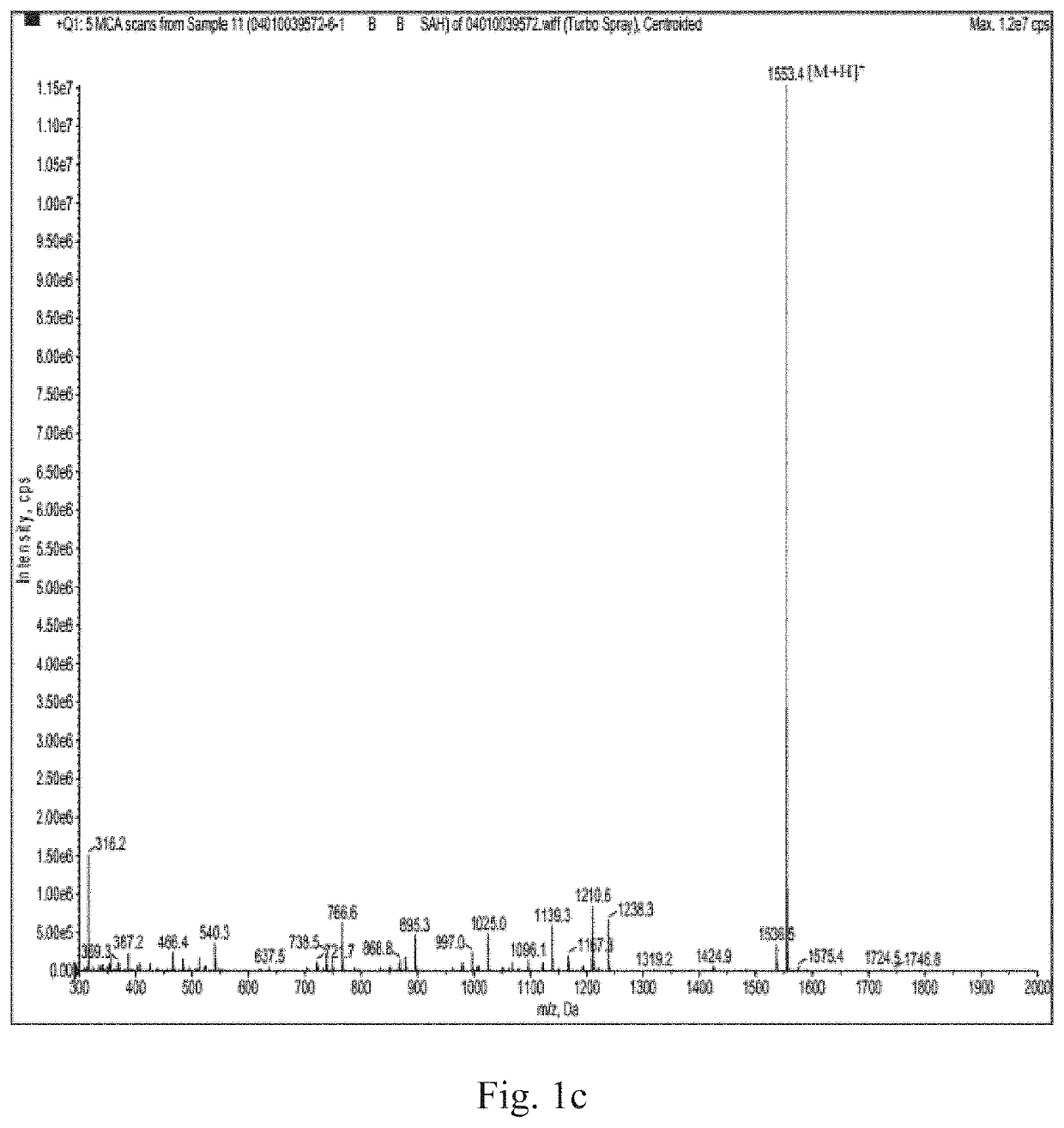
[0060]2) Solid-phase chemical synthesis, purity detection and molecular weight confirmation of sp2:
[0061]The solid-phase chemical synthesis of sp2 used the polypeptide Fmoc solid-phas...
example 2
Cell Proliferation Inhibition Test
[0064]Cells were digested and counted to prepare a cell suspension with a concentration of 1×105 cells / mL. 100 μL of the cell suspension was added to each well of a 96-well plate (1×104 cells per well); the 96-well plate was placed in a 37° C., 5% CO2 incubator for 24 h; 100 μL of the corresponding drug-containing medium was added to each well, and a negative control group, a menstruum control group, and a positive control group were set up at the same time with 5 replicate wells in each group; the 96-well plate was placed in a 37° C., 5% CO2 incubator for 72 h; 10 μL of CCK-8 solution was added to each well, and the culture plate was incubated in the incubator for 4 h; the OD value at 450 nm was measured with a microplate reader to calculate the inhibitory rate and IC50 value of sp2 on BxPC-3 tumor cell lines. The evaluation standard ploted different concentrations of the same sample versus the tumor cell inhibition rate to obtain a dose-effect cur...
example 3
[0065]In vivo efficacy evaluation of sp2 on human pancreatic cancer in situ, human cervical cancer, human ovarian cancer, human osteosarcoma, and human poorly differentiated pregastric cancer subcutaneously in nude mice[0066]I. Cell lines: human cervical cancer cell line SiHa, human ovarian cancer cell line A2780, human pancreatic cancer in situ BxPC-3, human poorly differentiated pregastric cancer BGC-823, and human osteosarcoma cell line MG-63 were cultured in the RPMI-1640 medium containing 10% fetal bovine serum.[0067]II. Modeling method: subcutaneous injection of cell line, tumor formation in the armpit[0068]III. Experimental steps:[0069](i)Cell Preparation Stage
[0070]1. The MG-63 cell lines in a good state of recovery was inoculated into a T75 cell culture flask and cultured at 37° C., 5% CO2.
[0071]2. The medium was changed every 2-3 days, and when the cell healing degree reached about 80%, a 1:3 passage was performed, and about 20 bottles were needed.
[0072]3. After the number...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com