Visible-light-induced direct oxidation method for saturated hydrocarbon bonds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
[0020]A visible-light-induced direct oxidation method for saturated hydrocarbon bonds, including the following specific steps:
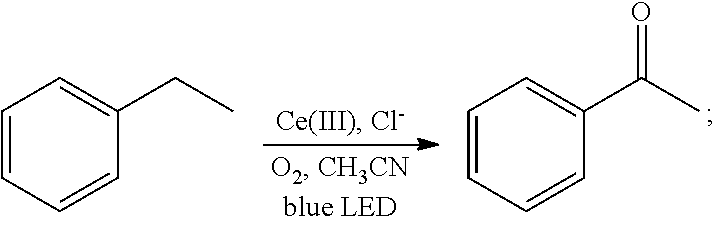
[0021]Oxygen was introduced into 2 mL of a solution of ethylbenzene (106 mg, 1 mmol) in acetonitrile for 20 min, until the solution was saturated with the oxygen. Then 1 mol % cerium complex cerium trichloride (2.4 mg, 0.01 mmol) and 2 mol % additive tetrabutylammonium chloride (5.5 mg, 0.02 mmol) were added. After the addition, the ethylbenzene and the oxygen were allowed to react for 2 hrs in the presence of the cerium complex and the additive at room temperature (25° C.) under the irradiation of a blue LED lamp (wavelength of 380 nm-550 nm). With the hydrocarbon bond in the ethylbenzene oxidized, the oxidation product acetophenone was thus acquired. The reaction was as follows:
[0022]After the reaction had stopped, the mixture was diluted with dichloromethane, washed sequentially with water and saturated brine, and dried. After separation by column chromato...
embodiment 2
[0023]A visible-light-induced direct oxidation method for saturated hydrocarbon bonds, including the following specific steps:
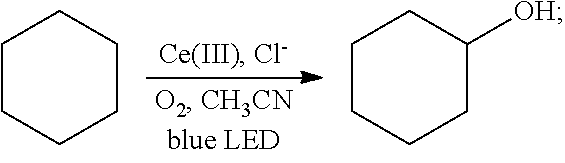
[0024]Oxygen was introduced into 2 mL of a solution of cyclohexane (85 mg, 1 mmol) in acetonitrile for 20 min, until the solution was saturated with the oxygen. Then 1 mol % cerium complex cerium trichloride (2.4 mg, 0.01 mmol) and 2 mol % additive tetrabutylammonium chloride (5.5 mg, 0.02 mmol) were added. After the addition, the cyclohexane and the oxygen were allowed to react for 5 hrs in the presence of the cerium complex and the additive at room temperature (25° C.) under the irradiation of a blue LED lamp (wavelength of 380 nm-550 nm). With the hydrocarbon bond in the cyclohexane oxidized, the oxidation product cyclohexanol was thus acquired. The reaction was as follows:
[0025]After the reaction had stopped, the mixture was diluted with dichloromethane, washed sequentially with water and saturated brine, and dried. After separation by column chromatograp...
embodiment 3
[0026]A visible-light-induced direct oxidation method for saturated hydrocarbon bonds, including the following specific steps:
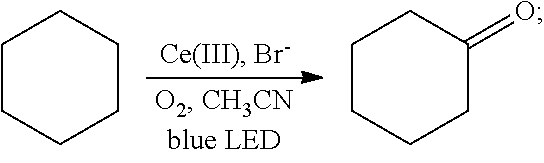
[0027]Oxygen was introduced into 2 mL of a solution of cyclohexane (85 mg, 1 mmol) in acetonitrile for 20 min, until the solution was saturated with the oxygen. Then 2 mol % cerium complex cerium nitrate (8.7 mg, 0.02 mmol) and 4 mol % additive tetrabutylammonium bromide (12.9 mg, 0.04 mmol) were added. After the addition, the cyclohexane and the oxygen were allowed to react for 48 hrs in the presence of the cerium complex and the additive at room temperature (25° C.) under the irradiation of a blue LED lamp (wavelength of 380 nm-550 nm). With the hydrocarbon bond in the cyclohexane oxidized, the oxidation product cyclohexanone was thus acquired. The reaction was as follows:
[0028]After the reaction had stopped, the mixture was diluted with dichloromethane, washed sequentially with water and saturated brine, and dried. After separation by column chromatography...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com